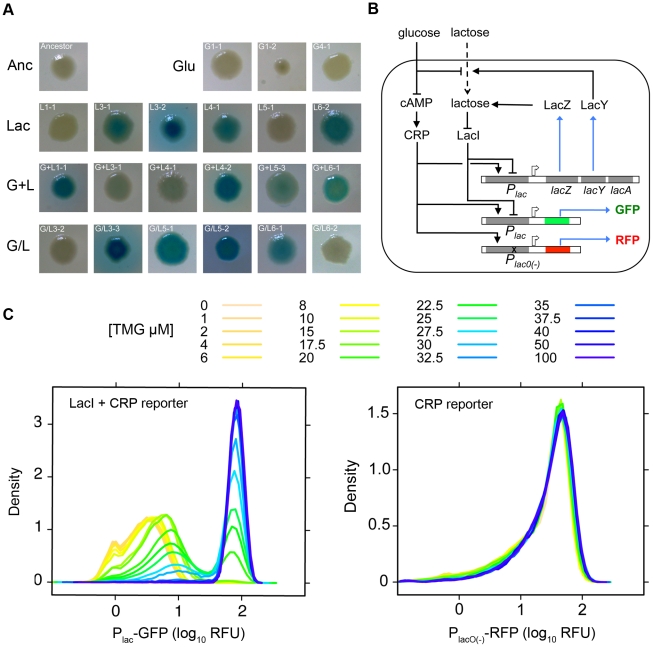
Figure 1. Characterization of lac operon regulation.
A) Examples of the range of evolved LacZ activity phenotypes present in the four evolution environments. Degree of blue coloration on TGX plates gives a qualitative measure of LacZ activity. B) Schematic of lac operon and reporters used to measure lac operon regulation. lacZ encodes the β-galactosidase responsible for lactose catabolism and lacY encodes a lactose permease. The expression of lacZYA is directly controlled by LacI and CRP. LacI is a negative regulator, binding to operator sites within the lacZYA promoter (Plac). LacI binding is inhibited by lactose and gratuitous inducers, such as TMG. CRP is a positive regulator, activating lacZYA expression when cAMP levels are elevated in response to low glucose concentrations. High levels of glucose also repress lac expression by inhibiting import of lactose through LacY. Two reporters were designed to measure LacI and CRP inputs into lac operon regulation. The native lac promoter drives expression of GFP and is subject to regulation by both LacI and CRP. A second reporter utilizes a mutant lac promoter that cannot bind LacI to drive expression of DsRedExpress2. This reporter is only subject to regulation by CRP. Solid lines indicate positive (arrows) and negative (blunt arrow) regulatory interactions; dotted lines indicate the transfer of metabolites; blue lines indicate the production of proteins; open arrows indicate expression start sites. Figure adapted from Ozbudak et al. 2004. C) Ancestral inducer response profile. Shown are flow cytometry histograms for the ancestor grown in a range of TMG concentrations. Plac-GFP and Plac(O-)-RFP measurements were taken simultaneously from the same cultures.