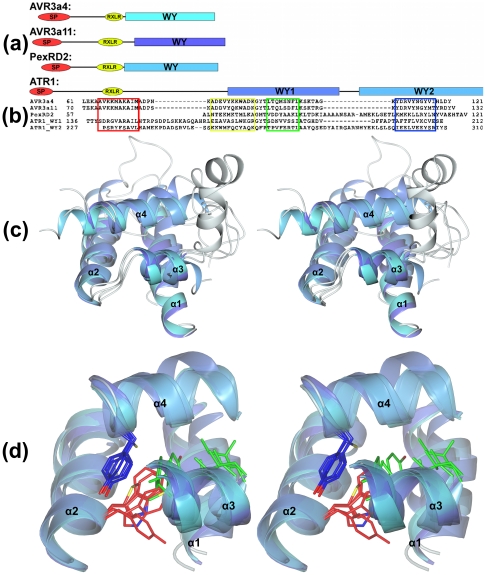
Figure 1. Structural conservation of the WY-domain fold in RXLR effectors from P. infestans, P. capsici, and H. arabidopsidis.
(a) Schematic representation (to scale) of the domain architectures of AVR3a4, AVR3a11, PexRD2, and ATR1 showing the positions of the WY-domains used in the structural overlays. SP = signal peptide region, RXLR = RXLR/dEER region, WY = WY-domain regions (schematics are aligned at the end of the RXLR/dEER region). (b) Structure-based sequence alignment showing the positions of the conserved helices in each WY-domain. (c) Stereo view of an overlay comprising the WY-domains from AVR3a11, ATR1-WY1, ATR1-WY2, PexRD2, and AVR3a4 (α2–α4 span the WY-domain, α1 is the N-terminal helix present in all but PexRD2). The helices of the structures are colored in grades of blue through to cyan. Connecting regions are in gray (all structures). (d) Stereo view (orientation as in (c)) showing the positions of important residues within the hydrophobic core of the WY-domain (red = the W position, blue = the Y position, in green are two positions contributed from α3). Only the conserved helices are shown in cartoon representation (connecting regions removed for clarity).