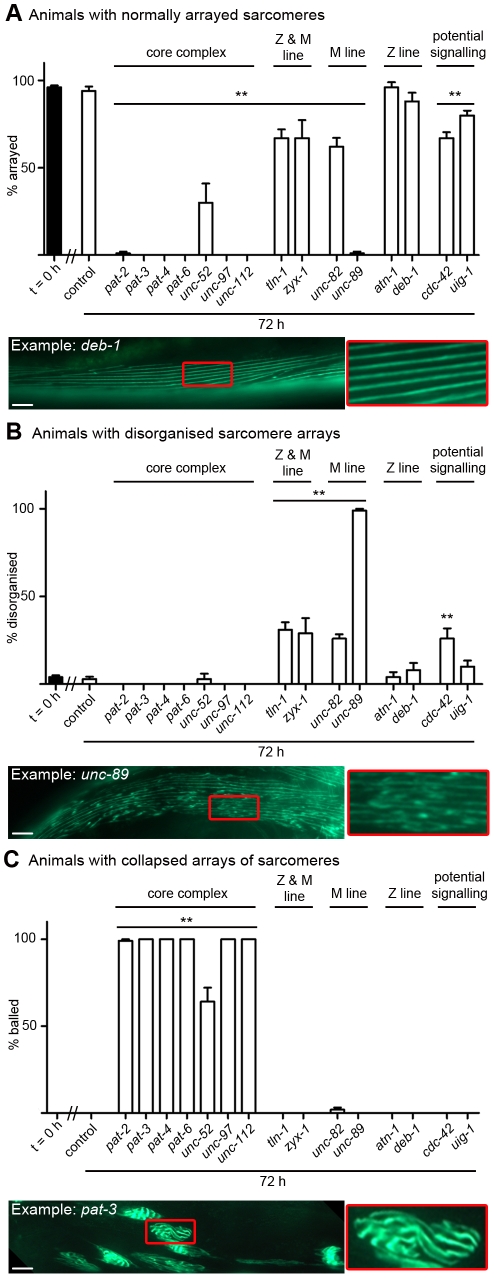
Figure 3. Acute loss of muscle attachment causes disorganisation and collapse of arrayed sarcomeres.
Animals expressing a full length translational fusion of gfp to myo-3 (myosin heavy chain A) were age synchronised at L1 stage and grown to young adulthood at 16°C (t = 0 h). Adult animals were then transferred to NGM RNAi plates [87] seeded with bacteria expressing dsRNA against genes indicated for a further 72 h to mid-adulthood. The 20 most Unc animals were picked and scored for identical defects in sarcomere structure in at least two muscles within the animal and this was repeated for 5 independent RNAi treatments (n = 100 animals per condition/time point). A) Percentage of animals where only normal arrays of sarcomeres were observed is displayed as average ± SEM. Below the graph is an example of an RNAi treated animal displaying normal arrays of sarcomeres (as indicated by straight parallel lines of GFP), these are enlarged 300% to the right of the micrograph. B) Percentage of animals where disorganisation of sarcomere arrays were observed is displayed as average ± SEM. Below the graph is an example of an RNAi treated animal displaying disorganised arrays of sarcomeres (as indicated by lack of straight parallel lines of GFP), these are enlarged 300% to the right of the micrograph. C) Percentage of animals where sarcomere arrays have collapsed into ball like structures is displayed as average ± SEM. Below the graph is an example of an RNAi treated animal displaying a collapsed array of sarcomeres (as indicated by parallel lines of GFP that are not straight, long, lines), these are enlarged 300% to the right of the micrograph. A sample image of an RNAi treatment against each gene can be found in Figure S1, not displayed here are animals with tears in the arrayed sarcomeres (see unc-52 in Figure S1). **Significant difference from control t = 72 h, P<0.001 (two way repeated measures ANOVA). Scale bars represent 15 µm.