Abstract
BACKGROUND
Transfusion-associated microchimerism (TA-MC), the persistence of significant levels of donor leukocytes in blood recipients for prolonged periods, has been demonstrated following non-leukoreduced and leukoreduced transfusion to patients with severe traumatic injury. Development of TA-MC has not been rigorously studied in settings that do not involve massive trauma where the blood is leukoreduced and irradiated.
STUDY DESIGN AND METHODS
A cohort of 409 prospectively followed medical and surgical adult and pediatric female recipients of leukoreduced and mostly irradiated allogeneic red blood cell and platelet transfusions were evaluated to determine development of TA-MC. Four and eight-week post-transfusion samples were analyzed using quantitative real-time polymerase chain reaction (RT-PCR) for Y-chromosome sequences in leukocyte DNA, the marker for microchimeric cells in female blood recipients. Repeat testing was performed on Y-chromosome positive samples to confirm microchimerism (MC), and subsequent post-transfusion samples were tested to investigate persistence of MC.
RESULTS
On initial testing, forty of 207 (19%) adult and forty-four of 202 (22%) pediatric female blood recipients demonstrated low level MC. On repeat testing of these and additional specimens, twelve (3%) recipients demonstrated low level transient MC, but none had persistent TA-MC similar to that seen in transfused trauma patients.
CONCLUSION
Persistence of MC was not demonstrated in adult and pediatric recipients of leukoreduced and mostly irradiated blood components. The risk of TA-MC appears to be dependent on the clinical setting and is rare other than in patients sustaining severe traumatic injury.
Keywords: microchimerism, transfusion, irradiation, pediatric, blood recipients
INTRODUCTION
Microchimerism (MC), the persistence of a small population (<5%) of non-self cells within an individual, has been observed in the settings of pregnancy (maternal-fetal MC),1 twinning,2 transplantation,3 and allogeneic blood transfusion.4,5 Persistence of donor leukocytes in blood recipients, designated transfusion-associated microchimerism (TA-MC), occurs in approximately 10% of patients transfused for severe traumatic injury, and its development is unaffected by leukoreduction.5,6 Although studies are limited, TA-MC has not been demonstrated in blood recipients receiving multiple transfusions for conditions such as human immunodeficiency virus infection,7 hemoglobinopathies,8 and elective orthopedic surgery.4
Passively transferred donor leukocytes in blood components are implicated in transfusion-related complications ranging from human leukocyte antigen (HLA) alloimmunization to transfusion-associated graft-versus-host disease (TA-GVHD) to cell-associated viral transmission and reactivation of latent recipient viral infections.9–11 Donor leukocytes may also modulate the host’s immune system with beneficial (e.g., enhanced tolerance to renal grafts)12 and possibly deleterious (e.g., increased risk of cancer recurrence and post-operative infections)13,14 clinical effects.15,16 To date, no long-term clinical consequences have been associated with TA-MC in trauma patients,6,17 although a carefully conducted case-control study to evaluate either clinical or laboratory stigmata of autoimmunity or chronic GVHD is lacking.
Development of persistent TA-MC has not been rigorously studied in settings other than massive trauma or where blood components are leukoreduced and gamma irradiated. Irradiation of allogeneic cellular blood components is considered an effective method of inhibiting the proliferative capacity of donor lymphocytes for prevention of TA-GVHD in susceptible high-risk patients.18 Similar to TA-MC, leukoreduction alone does not prevent the occurrence of TA-GVHD.19
We hypothesized that recipients of leukoreduced and irradiated cellular blood components would not demonstrate persistent TA-MC, which we believe represents hematopoietic stem cell engraftment.4,20 A large prospective study of transfusion-transmitted infections (TRIPS) in adult and pediatric allogeneic blood recipients of leukoreduced and predominantly gamma irradiated blood components afforded the opportunity to test this hypothesis. To determine the incidence and persistence of TA-MC, we first analyzed four- and eight-week post-transfusion blood samples of females enrolled in TRIPS using quantitative real-time polymerase chain reaction (RT-PCR) for detection of Y-chromosome. In patients with demonstrated MC, we determined if they had received male donor red blood cells (RBCs) or platelets (PLTs) and whether the component was gamma irradiated. Reserved aliquots of samples that initially tested positive for Y-chromosome and additional pre- and post-transfusion samples were tested to confirm and evaluate persistence of TA-MC.
MATERIALS AND METHODS
TRIPS patient population
In 2001, TRIPS began enrollment of adult and pediatric patients not transfused in the six weeks preceding the index transfusion (date of study enrollment). Recipients of allogeneic blood transfusion were enrolled at three centers in the Washington DC metropolitan area. Adult patients were enrolled at The National Institutes of Health (NIH) Clinical Center and The NIH Heart Center at Suburban Hospital, Bethesda, MD. Pediatric patients were enrolled at Children’s National Medical Center (CNMC), Washington DC. Recipient pre-, post-transfusion (four-, eight-, 12-, and 24-weeks), and donor whole blood specimens were collected and frozen for future analyses. All blood recipients received pre-storage leukoreduced blood components. All RBCs and PLTs transfused to adults were gamma irradiated (minimum of 25 Gy), whereas children received irradiated cellular blood components only for specific indications. This study was conducted in accordance with the Declaration of Helsinki, and all patients gave written informed consent or assent according to institutional guidelines. The study design was reviewed and approved by an Institutional Review Board (IRB) at each participating center. The de-identified analysis of blood specimens at Blood Systems Research Institute (BSRI) was approved under the purview of both NIH and CNMC IRBs.
TA-MC study design
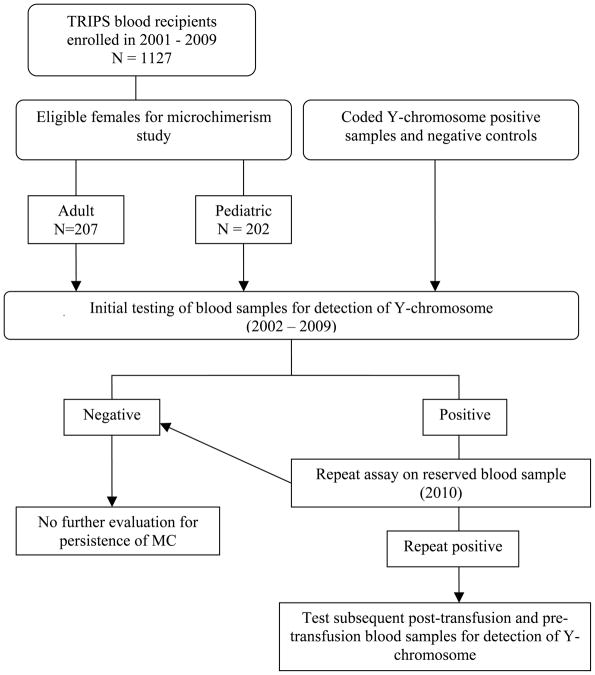
Between 2002 and 2009, four and eight-week post-transfusion frozen whole blood aliquots of non-pregnant and non-transplant TRIPS females were analyzed for the presence of Y-chromosome positive leukocytes indicative of TA-MC, using quantitative RT-PCR. To corroborate low-level detection of MC signal, coded Y-chromosome positive and negative control blood samples were tested in parallel with TRIPS case samples. Patient and control sample flow and the testing algorithm for microchimerism are summarized in Figure 1. Adult females evaluated for TA-MC were primarily medical service patients with underlying malignant diseases, whereas the pediatric females were primarily surgical patients with underlying cardiac, orthopedic or neurosurgical diagnoses. The median age was 51 years (range 20–86) and 7 years (range 0–21) for adult and pediatric patients, respectively. Demographic characteristics of study patients and post-transfusion samples available for initial testing of microchimerism are summarized in Table 1. When a female recipient demonstrated microchimerism on a four- and/or eight-week post-transfusion sample, donor and transfusion records were searched to determine if they had received male donor RBC or PLT components and the irradiation status. Blood donor gender was provided by blood bank staff or by blood suppliers for imported components. The storage age (days) of blood components was calculated from date of transfusion minus the date of collection. Female recipients with MC who received at least one male donor RBC or PLT component were considered possible cases of TA-MC and aliquots from the same sample date as well as other post-transfusion sample dates were tested to determine the reproducibility and duration of the finding.
Fig. 1.
Flowchart of patient allocation and sample handling from the time of enrollment to analysis of four and/or eight week post-transfusion blood samples for microchimerism.
TABLE 1.
Patient and post-transfusion sample characteristics for microchimerism testing
| Characteristic | Adult N = 207 |
Pediatric N = 202 |
||
|---|---|---|---|---|
| Patient diagnoses, n (%) | Malignancies | 167 (81%) | Cardiac | 111 (55%) |
| VHL* | 21 (10%) | Orthopedic | 52 (26%) | |
| Cardiac | 6 (3%) | SCD† | 26 (13%) | |
| Autoimmune | 5 (2%) | Neurosurgical | 7 (3%) | |
| Hematological | 5 (2%) | Trauma | 6 (3%) | |
| Immunodeficiency | 3 (1%) | |||
| Number (%) of patients with samples tested at indicated post-transfusion intervals | ||||
| 4-week | 41 (19.8%) | 85 (42%) | ||
| 8-week | 17 (8.2%) | 59 (29%) | ||
| 4- and 8-week | 149 (72%) | 58 (29%) | ||
VHL = von Hipple Lindau;
SCD = sickle cell disease
Sample processing for microchimerism analysis
Blood specimens were collected into ethylenediaminetetraacetate (EDTA)-anticoagulated tubes. Whole blood aliquots (one milliliter [mL]) were prepared at NIH and CNMC within 72 hours of phlebotomy and frozen without cryoprotectant for subsequent microchimerism testing at BSRI. Batched frozen aliquots were shipped frozen by overnight courier service to BSRI. All blood samples received a unique identifier that resulted in blind laboratory analysis with respect to the identity of the subject and their clinical history. The identifier allowed for subsequent linkage of the transfusion history, clinical history, and laboratory results for analysis.
Frozen whole blood aliquots (1 mL) were processed in batches. The blood was thawed and micro-centrifuged at 5000 RPM for 5 minutes. The supernatant was aspirated and residual RBCs in the WBC pellets lysed by the addition of 1 mL Solution A (0.1M KCl, 0.01M Tris Base, 0.0025M MgCl26H2O, pH 8.3) with 200 μL of Saponin lysis solution (0.4% Saponin in 0.5% NaCl, pH 7.4). The preparation was micro-centrifuged at 7000 RPM for 5 minutes, the supernatant aspirated, and the WBC pellet washed twice with 1 mL Solution A. A crude DNA lysate was prepared from this pellet by the addition of 250 μL of solution containing equal volumes of Solution A and Solution B (10mM Tris (pH8.3), 2.5mM MgCl26H2O, 1%Tween-20, 1% NP40), and 12.5 μg proteinase K (GibCo BRL, Carlsbad, California). Subsequently, the lysate solution was incubated at 60°C for 1.5 hours. Inactivation of proteinase K was followed by incubation at 95°C for 30 minutes.
Assessment of microchimerism
The detection and quantitation of chimerism by gene amplification requires an allogeneic marker unique to the chimeric cell population and an analytically sensitive assay.21 The use of allele-specific primer pairs that recognize at least a two- to three-base pair difference in the minor population allows for MC detection at levels as low as one chimeric cell per million host cells. In addition, real-time PCR is a powerful strategy in which the reaction products are observed at each cycle rather than at the reaction’s termination. With these PCR strategies, the detection of minor populations of donor cells is highly sensitive.21,22 We selected the Y-chromosome as the allogeneic marker for microchimeric cells in female blood recipients. Samples were also tested in parallel for HLA-DQα, a conserved region in the major histocompatibility complex (MHC) II locus, to establish input levels of human genomic DNA, which allowed for quantitation of male leukocytes relative to total recipient leukocytes in each sample.
PCR testing, Y-chromosome
From one mL whole blood samples, 250 μL DNA lysates were prepared and diluted 1:2, and 25 μL of this dilution was amplified in triplicate for Y-chromosome detection. This procedure is equivalent to amplification of 50 μL of whole blood per test (150 μL of whole blood per triplicate testing). If the residual DNA lysate was insufficient for this protocol, duplicate amplification replaced triplicate amplification. Duplicate reaction wells of male DNA (100 genomic equivalents (gEq)/reaction, 10 gEq/reaction, and 1 gEq/reaction) were amplified on each plate to determine standard curves. An allele-specific quantitative PCR assay for a 73-bp region of the sex-determining region of the human Y-chromosome sequence (SRY) was used as a marker for male MC. The detailed methodology and technical validation of these MC assays has been described previously.23 Briefly, 25 μL of DNA was added to 50 μL of buffer consisting of 1μM of each primer SB (5′ GAGGCGCAAGATGGCTCTAGAG 3′) and SC (5′ CCACTGGTATCCCAGCTGCTTGC 3′) (Integrated DNA Technologies, Coralville, IA), 6mM Magnesium, 25X of SYBR Green (FMC BioProducts, Rockland, ME), 1mM of dNTPs (Roche Diagnostics, Indianapolis, IN), 1.5 Units of FastStart enzyme per sample (Roche Diagnostics, Indianapolis, IN). RT-PCR was conducted using the following cycle conditions: 10 min @ 95°C followed by 45 cycles of 30 sec @ 95°C, 30 sec @ 68°C, and 45 sec @72°C. Dissociation curves of SRY amplicons were examined to confirm the specificity of the PCR reactions. All reagents were prepared and retained in a dedicated laboratory, separated from the sample preparation area.
PCR testing, HLA-DQα
Five μL of a 1:5 dilution of each crude DNA lysate was used to amplify HLA-DQα to quantify the total peripheral blood leukocyte genome equivalents in each test sample. A 242-base pair region of the HLA-DQα gene was amplified with primers GH26 (5′ GTGCTGCAGGTGTAAACTTGTACCAG 3′) and GH27 (5′ CACGGATCCGGTAGCAGCGGTAGAGTTG 3′), 5mM magnesium and the following cycling conditions: 10 min @ 95°C followed by 45 cycles of 30 sec @ 95°C, 30 sec @ 56°C and 45 sec @ 72°C. This is equivalent to 4 μL of whole blood (8 μL of whole blood amplified in duplicate). Standard curves for quantitation were established by inclusion of duplicate reaction wells containing known quantities of DNA (1000 gEq/reaction, 100 gEq/reaction, and 10 gEq/reaction) on each plate. Dissociation curves of HLA-DQα amplicons were examined to assess specificity.
Interpretation of MC results and statistical analyses
There are other potential sources of microchimerism aside from transfusions given during the study period that may have been responsible for inducing MC in female patients. These include fetal-maternal MC (bidirectional trafficking of maternal and fetal cells during pregnancy), twinning (exchange of cells between fraternal twins), past male donor derived transfusions, or detection of delayed clearance of donor leukocytes (i.e., transient MC). In addition when the minor leukocyte population is present at very low levels, detection of MC may become stochiastic (microchimeric cells may or may not be present in the sample). These factors were considered in the interpretation of post-transfusion MC results of female blood recipients. Pregnancy history of adult females was searched by review of medical records. The timing of transfusions given during the study period relative to blood sampling for MC testing was reviewed.
The Y-chromosome copy number was normalized against total recipient leukocytes based on the HLA-DQα results. The data are expressed as a ratio of Y-chromosome copies (MC male cells) per 10,000 total cells. We considered the recipient to have confirmed MC if repeat testing of reserved frozen aliquots for Y-chromosome yielded reproducible results. We compared the total genomic input of blood samples testing positive and negative for Y-chromosome sequences to ensure that there was no difference in genomic input that would influence the detection of MC. Statistical analysis utilized the Student’s t-test by software R/Bioconductor and differences of p <0.05 were considered significant.
To assess the susceptibility of pediatric female patients transfused with either non-irradiated or irradiated male donor RBCs or PLTs for development of MC, transfusion records for all pediatric patients were searched to determine irradiation status of male donor components transfused during the study period. Statistical analysis utilized the Fisher’s exact test and differences of p <0.05 were considered significant.
RESULTS
Validation of microchimerism assay
Coded Y-chromosome positive and negative control blood samples were tested in parallel with female TRIPS case samples. Frozen whole blood samples from 15 males (20 samples), 8 transplanted females (15 samples), and two nulliparous and non-transfused females (46 distinctly coded negative control samples) yielded appropriate test results in all cases: Y-chromosome was undetectable in samples from females transplanted with female donor hematopoietic stem cells (HSCs) and negative control females, whereas blood samples from males and females transplanted with male donor HSCs demonstrated high levels of MC (male leukocytes/10,000 total leukocytes ranged from 461 to 25,827 and 100 to 64,313, respectively).
Initial and repeat testing for microchimerism in adult and pediatric blood recipients
On initial testing, 44 of 356 adult (12.3%) and 47 of 260 pediatric (18%) four- and/or eight-week post-transfusion blood samples demonstrated low level Y-chromosome signal. The number of microchimeric cells per 10,000 total cells ranged from 0.01 to 72.56 (0.0001% to 0.73%) in adult samples and from 0.03 to 28.57 (0.0003% to 0.29%) in pediatric samples. There was no difference in the total genomic input, measured by HLA-DQα PCR, between blood samples that tested positive and negative for Y-chromosome (p-value>0.05) (Table 2). To confirm microchimerism, repeat assays were performed on reserve frozen aliquots of four- and eight-week samples that had tested positive by Y-chromosome PCR. The repeat assay confirmed low level microchimerism in five adult and seven pediatric samples, representing 1.4% and 2.7%, respectively, of the TRIPS samples initially tested.
TABLE 2.
Leukocyte input of post-transfusion blood samples that initially tested positive or negative for Y-chromosome indicating possible TA-MC
| Characteristic | Adult | Pediatric |
|---|---|---|
| Samples tested, n | 356 | 260 |
| Y-chromosome positive, n (%) | 44 (12%) | 47 (18%) |
| Leukocyte input* | 3,565,325 ± 4,596,723† | 2,827,685 ± 452,1795† |
| Y-chromosome negative, n (%) | 312 (88%) | 213 (82%) |
| Leukocyte input* | 5,124,264 ± 7,809,331 | 2,678,921 ± 4,520,954 |
Data are given as mean ± SD of HLA-DQα PCR-based human genome equivalents per mL of whole blood.
p-value by t-test statistics comparing the Y-chromosome positive and negative groups was not significant.
Of the 40 adult recipients (44 post-transfusion blood samples) of leukoreduced and irradiated cellular blood components who tested positive for Y-chromosome on initial testing, 39 were transfused with at least one male donor RBC or PLT component and the other recipient received two female donor components during the study period. On repeat testing, five recipients had reproducible low level microchimerism. Forty-four pediatric recipients (47 post-transfusion blood samples) of leukoreduced and selectively irradiated RBCs or PLTs demonstrated low level MC on initial testing. Of these recipients, 15 were not transfused with male donor RBCs or PLTs; seven were transfused with irradiated male donor components; 16 were transfused with non-irradiated male donor RBCs or PLTs, and six received both irradiated and non-irradiated male donor components (Table 3). On repeat testing, two females not transfused with male donor cellular components and five females transfused with non-irradiated male donor components had reproducible low level MC. There was no significant difference in rate of detection of initially reactive Y-chromosome signal in patients who received exclusively irradiated male donor RBCs or PLTs, compared to those who received non-irradiated male donor components (p-value >0 .05) (Table 3).
TABLE 3.
Transfusion characteristics (male unit exposures and irradiation status) for pediatric female patients tested for Y-chromosome to detect TA-MC
| Y-Chromosome positive, n (%) | |||
|---|---|---|---|
| Transfusion Status | Patients, n | Initial* | Repeat† |
| Received no male donors | 54 | 15 (28%) | 2 (4%) |
| Received only IRR male blood | 27 | 7 (26%) | 0 |
| Received only non-IRR male blood | 92 | 16 (17%) | 3 (3%) |
| Received IRR & non-IRR male blood | 29 | 6 (21%) | 2 (7%) |
Y-chromosome positive on initial test, but retained aliquot tested negative.
Y-chromosome positive on initial test and on repeat test of a retained aliquot.
Patients were divided in two groups, according to receipt of IRR or non-IRR male donor cellular blood components, and presence of microchimerism was compared between the two groups using the Fisher exact test (p value = 0.42).
IRR = irradiated.
Transfusion and clinical characteristics of patients with repeatable MC
Twelve female blood recipients demonstrated repeatable low level microchimerism in four- and/or eight-week post-transfusion samples (Table 4). One patient (recipient C) had a repeat positive four-week post-transfusion sample, but no subsequent post-transfusion samples were available for analysis. Testing of pre-transfusion and/or subsequent post-transfusion blood samples to investigate the source and persistence of MC was therefore undertaken for five recipients (Table 4). A single patient (recipient F) tested negative pre-transfusion and then demonstrated low level microchimerism in both the four- and 24-week post-transfusion samples; no intervening samples were available for testing. Three patients (recipients E, G, and J) were MC positive in the eight-week, but not in the 12-week post-transfusion sample; recipient J was negative pre-transfusion, but no pre-transfusion sample was available for recipients E and G. One patient (recipient I) who was negative pre-transfusion had a repeat positive eight-week post-transfusion sample, but no subsequent post-transfusion samples were available for testing.
TABLE 4.
Transfusion and microchimerism characteristics of patients with repeatable low level microchimerism
| Male leukocytes / 10000 recipient leukocytes, initial / repeat results | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Components transfused*, n | Male donor blood, n (Non-IRR, n) | Pre-transfusion | Post-transfusion | |||
| Pediatric | 4-wks | 8-wks | 12-wks | 24-wks | |||
| A | 4 | 4 (3) | 4.3 / 3.2 | 0 / NA† | |||
| B | 3 | 0 (0) | 1.7 / 3.2 | 0 / NA | |||
| C | 4 | 3 (2) | NS§ | 0.03 / 7.5 | NS | NS | NS |
| D | 1 | 0 (0) | 3.03 / 0.7 | 2.9 / 0 | |||
| E | 2 | 1 (1) | NS | 0 / NA | 28.6 / 1.6 | 0 | NA |
| F | 5 | 1 (1) | 0 | 1.01 / 0.5 | NS | NS | 0.2 |
| G | 2 | 2 (2) | NS | NS | 0.5 / 1.3 | 0 | NA |
| Adult | |||||||
| H | 7 | 5 (0) | 0.02 / 0.4 | 0 / NA | |||
| I | 6 | 3 (0) | 0 | 0 / NA | 0.06 / 0.5 | NS | NS |
| J | 20 | 10 (0) | 0 | 0 / NA | 0.6 / 6.9 | 0 | NA |
| K | 9 | 7 (0) | 0.1 / 0.5 | 0 / NA | |||
| L | 10 | 4 (0) | 0.03 / 0.4 | 0.03 / 0 | |||
Total number of components transfused from study entry up to collection of the 8-week post-transfusion blood sample.
NA = not applicable;
NS = no sample.
The median number of total blood components transfused to adult female patients with repeatable MC (recipients H-L) was nine (range 6–20); the median number of irradiated male donor RBCs or PLTs transfused was five (range 3–10) (Table 4). In contrast, the median number of total blood components transfused to pediatric females (recipients AG) was three (range 1–5) (Table 4). Two pediatric patients appeared to demonstrate low level MC despite having received either no male donor blood components (recipient B) or only male donor plasma (recipient D) at the time of entry into the study. Five children with repeatable MC were transfused with non-irradiated male donor cellular blood components (range 1–3); of these blood recipients, two (recipient A and C) were transfused with RBCs of less than seven days of storage age. The interval between transfusion and collection of post-transfusion blood samples was more than 14 days in all cases except for one patient (recipient H) who received a male donor blood component 11 days prior to the four-week post-transfusion blood sample collection.
In those with repeatable low level microchimerism, the median patient age was 53 years (range 42–60) and 61.4 months (range 7.4–186) for adult and pediatric cases, respectively. Three of the seven pediatric patients (recipients A–C) had underlying cardiovascular primary diagnoses but none were associated with either DiGeorge or Wiskott-Aldrich syndrome, or demonstrated clinical manifestations of congenital disorders of defective immunity. One patient with an endocardial cushion defect also had Down’s syndrome, which is associated with abnormalities of both the innate and adaptive immune system.24 Two pediatric patients (recipients F and G) had sickle cell disease as the primary diagnosis. One patient had an underlying spinal cord anomaly and neurofibroma. All five adult patients (recipients H-L) had underlying malignant diagnoses (peritoneal mesothelioma, T-cell lymphoma, two patients with metastatic melanoma, and Von Hipple-Lindau (VHL) syndrome with renal cell carcinoma) and none had a primary immunodeficiency disorder. Four adult patients were treated with chemotherapy or total body irradiation prior to or during the study period. The patient with VHL syndrome underwent a partial nephrectomy for renal cell carcinoma. Pregnancy history, including gender of children, was not available for any of the adult female patients.
DISCUSSION
The incidence and persistence of TA-MC has not been systematically studied in clinical settings that do not involve massive trauma or where blood components are both leukoreduced and irradiated. Development of persistent high level microchimerism was not demonstrated in any of 409 medical and surgical adult or pediatric recipients of leukoreduced and predominantly gamma-irradiated cellular blood components evaluated in this study. However, twelve of 409 (3%) female blood recipients demonstrated transient low level (<1%) microchimerism (MC male cells) at four or eight weeks post-transfusion.
These results are in contrast to the consistent finding of high level persistent TA-MC in approximately 10% of blood recipients transfused for severe traumatic injury.4,17 In the adult trauma setting, donor leukocytes have been detected for months to years post-transfusion at levels as high as 3–5% of the recipients’ total circulating leukocytes, even in recipients of leukoreduced blood products.5,6 The degree of short-term immune suppression associated with severe trauma,25 predisposing genetic factors,26 and the magnitude of donor-recipient immunologic reactivity correlate with the development of TA-MC in trauma recipients.20
Currently, it is not known whether the persistence of donor leukocytes represents engraftment at the level of the hematopoietic peripheral blood progenitor cell or proliferation of donor T-lymphocytes. The observations that support the hematopoietic engraftment hypothesis include the long duration of persistent donor cells, the increasing number of cells over time, and the presence of multiple immunophenotypic lineages.4,20 Given that leukoreduction alone does not prevent development of TA-MC, it appears that the number of transfused donor cells is less important than their capacity to proliferate.6 Gamma irradiation of cellular blood components is an effective method of inhibiting the proliferative capacity of donor lymphocytes for prevention of TA-GVHD, a rare but fatal transfusion complication.27 Similar to TA-MC, leukoreduction of blood alone does not prevent the development of TA-GVHD in high-risk patients.19 Patients at risk for TA-GVHD include premature infants and those with congenital immunodeficiency, hematological malignancy or solid tumors undergoing ablative chemotherapy or radiotherapy, transfusion of HLA-matched products or familial blood donations, and treatment with fludarabine.10 In this study, some recipients of leukoreduced and irradiated male donor RBCs and/or PLTs demonstrated extremely low level microchimerism, but none developed persistent microchimerism. The low levels of MC observed in recipients of exclusively irradiated male donor blood components and those who did not receive male donor components likely represent false positives (i.e., background noise of the assay), however, transient low level MC can not be ruled out. Overall, it appears that adult and pediatric recipients of leukoreduced and irradiated blood components are not at risk for development of persistent TA-MC, even if severely immunocompromised.
The low level detection of male cells demonstrated in this study population could reflect non-specificity at the assay’s lower limit of detection or represent stochastic detection of extremely low levels of male leukocytes present in the limited sample volumes processed for analysis in a single PCR reaction. Larger samples of blood would need to be analyzed in multiple parallel aliquots under identical reaction conditions in order to overcome the problem of stochastic detection.28 Other possible sources of low level MC include previous male pregnancies (feto-maternal MC),1 twinning (exchange of cells between fraternal twins),2 or transient male donor leukocytes related to the timing of transfusion and collection of post-transfusion blood samples. The latter explanation is less likely given that collection of post-transfusion blood samples for all cases was greater than two weeks after transfusion(s) except for one case in which the interval was 11 days and that blood component was irradiated. In previous studies of short-term kinetics in orthopedic surgical patients, donor leukocytes transiently increase at three to five days following transfusion and then are cleared from the recipient’s circulation by approximately two weeks.29,30 However, the life span of blood lymphocytes is probably weeks to years.31 It is possible that the low level detection of MC in patients not transfused with male donor blood components during the study period may represent long-lived non-proliferating cells from prior transfusions derived from male donors. The pre-transfusion samples of three of the 12 cases who demonstrated repeatable low level MC were negative for Y-chromosome. One recipient demonstrated low level MC at 24-weeks post-transfusion, the receipt of other male donor-derived transfusion(s) after the index transfusion could not be excluded, thus confounding the distinction between persistent MC and a second episode of transient MC.
The development of stable MC in pediatric blood recipients is of particular clinical importance given that transfused children are expected to survive to adulthood and are more likely to suffer the long-term consequences of transfusion.32,33 Establishing whether persistent TA-MC occurs in children, and if so, defining its long-term clinical consequences (i.e., GVHD or autoimmune diseases) would require large studies of long duration. Children with congenital blood disorders (e.g., thalassemia, sickle cell disease, Diamond-Blackfan anemia) are often managed with chronic red blood cell transfusions, in some instances, for a lifetime. Young pediatric patients, in particular newborns and premature infants, are potentially at increased risk for the development of TA-MC because of an incompletely developed immune system34 and frequent transfusion with blood of shorter storage age (≤ 14 days), which contains viable donor leukocytes. There is no universally accepted “standard of practice” for transfusion of irradiated blood in children and practices vary nationally and internationally.35,36 Many tertiary-care hospitals provide irradiated blood components to all infants until they reach a predetermined age, often around one year of age to avoid overlooking infants with undiagnosed cellular primary immunodeficiency diseases.36 Failures to irradiate when clinical indicated or to identify patients at risk for TA-GVHD occur.37 The true incidence of TA-GVHD is unknown and may be obscured by misdiagnosis and underreporting.38 Recently, one group has suggested that all children under the age of six should receive irradiated components for prevention of TA-GVHD; the authors report success with implementation of this protocol and both costs and risks of irradiated components were minimal at their institution.38
In the current study, pediatric recipients were predominantly surgical patients, and only selected patients were transfused with gamma irradiated cellular blood components if clinically indicated for prevention of TA-GVHD. Of the 111 pediatric cardiovascular patients tested for MC, 25 patients demonstrated transient MC and three had repeatable low level MC that was not persistent in the two where subsequent post-transfusion samples were available for testing. This finding is consistent with a previous investigation by our group that demonstrated transient donor leukocyte persistence in pediatric patients receiving transfusion for structural heart disease (unpublished data). Pediatric recipients of non-irradiated male donor RBCs and/or PLTs were not at increased risk for development of MC compared to recipients of exclusively irradiated male donor blood components. Of the 121 pediatric female recipients of non-irradiated male donor components, five (4%) demonstrated repeatable low level Y-chromosome signal that was not persistent in the three where subsequent post-transfusion samples were available for testing. However, two of 54 (4%) pediatric patients who did not receive male donor RBCs or PLTs also had repeatable low level detection of Y-chromosome sequences indicating other sources of MC (e.g., twinning) or assay non-specificity.
To our knowledge, no large study has compared the frequency of MC in recipients of non-irradiated and irradiated blood with serial post-transfusion blood samples. A study of post-transfusion immune modulation in premature infants by Wang-Rodriguez et al investigated MC using semi-quantitative PCR for Y-chromosome detection in six female infants who received male donor blood.39 In this small study, two of three female infants transfused with non-leukoreduced and irradiated blood demonstrated male donor leukocytes only in the sample obtained one day post-transfusion; samples collected prior to transfusion and five to 14 days post-transfusion did not contain male donor leukocytes. Male donor leukocytes were not detected in three female infants who received leukoreduced and irradiated blood.39 In contrast, persistence of donor leukocytes has been demonstrated in recipients after fetal or neonatal transfusion with non-leukoreduced and non-irradiated blood, especially following intrauterine transfusion.40,41 It is noteworthy that in these settings of intrauterine and neonatal transfusion, persistent MC occurred without the development of GVHD or other discernable MC-associated illness at time of study. While other factors may contribute to the development of TA-MC, it would appear that the pediatric patients in this study were not at increased risk for persistent MC despite transfusion of non-irradiated and shorter storage age blood components.
Of interest are pediatric patients who are on chronic transfusion protocols. This study included twenty-six patients with sickle cell disease (SCD). A total of four SCD patients demonstrated low level MC on initial testing, and of these, two patients transfused with non-irradiated blood components demonstrated repeatable MC. Marshall et al. evaluated ten hemoglobinopathy patients (i.e., SCD and thalassemia) for microchimerism; four patients (three with SCD and one with thalassemia) demonstrated transient TA-MC 22 to 36 days post-transfusion.42 Four of eight blood recipients of non-irradiated components demonstrated microchimerism, but duration was unknown in two recipients.42 Similar to our findings, it appears that SCD patients commonly demonstrate transient MC. Determining the prevalence, persistence, and clinical significance (i.e., autoimmunity) of MC in hemoglobinopathy patients, could have potential implications for establishing transfusion practice policies and clinical management of chronically transfused patients.
Of note, six pediatric trauma patients were evaluated for TA-MC in the current study. On initial testing, two patients demonstrated low level MC that was not reproducible on repeat testing. Pediatric trauma patients should be considered for further investigation of TA-MC given the markedly diminished immune response that occurs transiently following severe injury (e.g. septic shock, extensive burns and tissue injury).43 Such a study should include varying pediatric age groups to investigate the clinical correlates and immunologic mechanisms associated with development of TA-MC, which will lead to a better understanding of the rate at which pediatric patients develop immune competence.
We used quantitative RT-PCR for the detection of Y-chromosome to investigate the incidence and persistence of TA-MC in female recipients of allogeneic blood components. The limitation of utilizing this strategy is that negative Y-chromosome test results cannot determine whether microchimerism from female allogeneic sources may exist. Lee and co-workers have developed and validated both HLA and non-HLA (insertion/deletion polymorphisms) based quantitative RT-PCR methods specifically for detection of TA-MC that is gender independent (sensitivity of 96% and specificity approaching 100%).44 This approach requires large volumes of blood samples for parallel PCR amplifications using allele specific probes that were not available for the present study. However, the absence of persistent MC based on Y-chromosome PCR in any subject in this study makes it unlikely that gender independent MC assays would yield a significantly different study outcome.
In conclusion, we demonstrate that TA-MC is rare in medical and surgical adult and pediatric recipients of leukoreduced and irradiated blood components. The risk of TA-MC therefore appears to be strongly dependent on the clinical setting of severe injury and is infrequent in other transfusion contexts.
Acknowledgments
We thank Herbert Perkins, MD and Margaret Clark, PhD for their thoughtful comments on earlier versions of this manuscript. We thank Joan Gibble, MD, Medical Director, Biomedical Services American Red Cross (ARC) and staff for providing blood donor gender data of ARC components. We thank the NHLBI Biorepository for their assistance with long-term storage of blood samples in the TRIPS/TRIPPS studies. We thank Shiquan Wu, PhD for assistance with statistical analyses.
This work was supported by Grant R01HL67229 from the National Heart, Lung, and Blood Institute, National Institutes of Health.
Footnotes
REPRINTS WILL NOT BE AVAILABLE
Conflict of Interest: None
References
- 1.Bianchi DW, Zickwolf GK, Weil GJ, et al. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996;93:705–8. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunsford I, Bowley CC, Hutchison AM, et al. A human blood-group chimera. Br Med J. 1953;2:80–1. doi: 10.1136/bmj.2.4827.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams KM, Nelson JL. Microchimerism: an investigative frontier in autoimmunity and transplantation. JAMA. 2004;291:1127–31. doi: 10.1001/jama.291.9.1127. [DOI] [PubMed] [Google Scholar]
- 4.Lee TH, Paglieroni T, Ohto H, et al. Survival of donor leukocyte subpopulations in immunocompetent transfusion recipients: frequent long-term microchimerism in severe trauma patients. Blood. 1999;93:3127–39. [PubMed] [Google Scholar]
- 5.Lee TH, Paglieroni T, Utter GH, et al. High-level long-term white blood cell microchimerism after transfusion of leukoreduced blood components to patients resuscitated after severe traumatic injury. Transfusion. 2005;45:1280–90. doi: 10.1111/j.1537-2995.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- 6.Utter GH, Nathens AB, Lee TH, et al. Leukoreduction of blood transfusions does not diminish transfusion-associated microchimerism in trauma patients. Transfusion. 2006;46:1863–9. doi: 10.1111/j.1537-2995.2006.00991.x. [DOI] [PubMed] [Google Scholar]
- 7.Kruskall MS, Lee TH, Assmann SF, et al. Survival of transfused donor white blood cells in HIV-infected recipients. Blood. 2001;98:272–9. doi: 10.1182/blood.v98.2.272. [DOI] [PubMed] [Google Scholar]
- 8.Reed W, Lee TH, Vichinsky EP, et al. Sample suitability for the detection of minor white cell populations (microchimerism) by polymerase chain reaction. Transfusion. 1998;38:1041–5. doi: 10.1046/j.1537-2995.1998.38111299056314.x. [DOI] [PubMed] [Google Scholar]
- 9.Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. The Trial to Reduce Alloimmunization to Platelets Study Group. N Engl J Med. 1997;337:1861–9. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- 10.Ruhl H, Bein G, Sachs UJ. Transfusion-associated graft-versus-host disease. Transfus Med Rev. 2009;23:62–71. doi: 10.1016/j.tmrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Busch MP, Collier A, Gernsheimer T, et al. The Viral Activation Transfusion Study (VATS): rationale, objectives, and design overview. Transfusion. 1996;36:854–9. doi: 10.1046/j.1537-2995.1996.361097017169.x. [DOI] [PubMed] [Google Scholar]
- 12.Opelz G, Sengar DP, Mickey MR, Terasaki PI. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc. 1973;5:253–9. [PubMed] [Google Scholar]
- 13.Vamvakas EC. Transfusion-associated cancer recurrence and postoperative infection: meta-analysis of randomized, controlled clinical trials. Transfusion. 1996;36:175–86. doi: 10.1046/j.1537-2995.1996.36296181932.x. [DOI] [PubMed] [Google Scholar]
- 14.Vamvakas EC. Meta-analysis of randomized controlled trials comparing the risk of postoperative infection between recipients of allogeneic and autologous blood transfusion. Vox Sang. 2002;83:339–46. doi: 10.1046/j.1423-0410.2002.00230.x. [DOI] [PubMed] [Google Scholar]
- 15.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327–48. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion-associated immunomodulation: fact or fiction? Blood. 2001;97:1180–95. doi: 10.1182/blood.v97.5.1180. [DOI] [PubMed] [Google Scholar]
- 17.Utter GH, Lee TH, Rivers RM, et al. Microchimerism decades after transfusion among combat-injured US veterans from the Vietnam, Korean, and World War II conflicts. Transfusion. 2008;48:1609–15. doi: 10.1111/j.1537-2995.2008.01758.x. [DOI] [PubMed] [Google Scholar]
- 18.Fearon T, Criss VR, Luban NL. Blood irradiator dosimetry with BANG polymer gels. Transfusion. 2005;45:1658–62. doi: 10.1111/j.1537-2995.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 19.Akahoshi M, Takanashi M, Masuda M, et al. A case of transfusion-associated graft-versus-host disease not prevented by white cell-reduction filters. Transfusion. 1992;32:169–72. doi: 10.1046/j.1537-2995.1992.32292180149.x. [DOI] [PubMed] [Google Scholar]
- 20.Utter GH, Owings JT, Lee TH, et al. Microchimerism in transfused trauma patients is associated with diminished donor-specific lymphocyte response. J Trauma. 2005;58:925–31. doi: 10.1097/01.ta.0000162142.72817.5c. discussion 31–2. [DOI] [PubMed] [Google Scholar]
- 21.Reed WF, Lee TL, Trachtenberg E, et al. Detection of microchimerism by PCR is a function of amplification strategy. Transfusion. 2001;41:39–44. doi: 10.1046/j.1537-2995.2001.41010039.x. [DOI] [PubMed] [Google Scholar]
- 22.Utter GH, Reed WF, Lee TH, Busch MP. Transfusion-associated microchimerism. Vox Sang. 2007;93:188–95. doi: 10.1111/j.1423-0410.2007.00954.x. [DOI] [PubMed] [Google Scholar]
- 23.Ariga H, Ohto H, Busch MP, et al. Kinetics of fetal cellular and cell-free DNA in the maternal circulation during and after pregnancy: implications for noninvasive prenatal diagnosis. Transfusion. 2001;41:1524–30. doi: 10.1046/j.1537-2995.2001.41121524.x. [DOI] [PubMed] [Google Scholar]
- 24.Bloemers BL, van Bleek GM, Kimpen JL, Bont L. Distinct abnormalities in the innate immune system of children with Down syndrome. J Pediatr. 2010;156:804–9. 9, e1–9, e5. doi: 10.1016/j.jpeds.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Giannoudis PV. Current concepts of the inflammatory response after major trauma: an update. Injury. 2003;34:397–404. doi: 10.1016/s0020-1383(02)00416-3. [DOI] [PubMed] [Google Scholar]
- 26.Gill RM, Lee TH, Utter GH, et al. The TNF (-308A) polymorphism is associated with microchimerism in transfused trauma patients. Blood. 2008;111:3880–3. doi: 10.1182/blood-2007-08-107144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelszynski MM, Moroff G, Luban NL, et al. Effect of gamma irradiation of red blood cell units on T-cell inactivation as assessed by limiting dilution analysis: implications for preventing transfusion-associated graft-versus-host disease. Blood. 1994;83:1683–9. [PubMed] [Google Scholar]
- 28.Dzik S. Principles of counting low numbers of leukocytes in leukoreduced blood components. Transfus Med Rev. 1997;11:44–55. doi: 10.1016/s0887-7963(97)80009-0. [DOI] [PubMed] [Google Scholar]
- 29.Schechter GP, Soehnlen F, McFarland W. Lymphocyte response to blood transfusion in man. N Engl J Med. 1972;287:1169–73. doi: 10.1056/NEJM197212072872303. [DOI] [PubMed] [Google Scholar]
- 30.Lee TH, Donegan E, Slichter S, Busch MP. Transient increase in circulating donor leukocytes after allogeneic transfusions in immunocompetent recipients compatible with donor cell proliferation. Blood. 1995;85:1207–14. [PubMed] [Google Scholar]
- 31.Price TH. Neutrophil and Lymphocyte Kinetics. In: Rossi EC, Simon Toby L, Moss Gerald S, editors. Principles of Transfusion Medicine. Baltimore: Williams and Wilkins; 1999. pp. 281–2. [Google Scholar]
- 32.Kamper-Jorgensen M, Ahlgren M, Rostgaard K, et al. Survival after blood transfusion. Transfusion. 2008;48:2577–84. doi: 10.1111/j.1537-2995.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 33.Gauvin F, Champagne MA, Robillard P, et al. Long-term survival rate of pediatric patients after blood transfusion. Transfusion. 2008;48:801–8. doi: 10.1111/j.1537-2995.2007.01614.x. [DOI] [PubMed] [Google Scholar]
- 34.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 35.Spinella PC, Dressler A, Tucci M, et al. Survey of transfusion policies at US and Canadian children’s hospitals in 2008 and 2009. Transfusion. 2010;50:2328–35. doi: 10.1111/j.1537-2995.2010.02708.x. [DOI] [PubMed] [Google Scholar]
- 36.New HV, Stanworth SJ, Engelfriet CP, et al. Neonatal transfusions. Vox Sang. 2009;96:62–85. doi: 10.1111/j.1423-0410.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 37.Neves JF, Marques A, Valente R, Barata D. Nonlethal, attenuated, transfusion-associated graft-versus-host disease in an immunocompromised child: case report and review of the literature. Transfusion. 2010;50:2484–8. doi: 10.1111/j.1537-2995.2010.02705.x. [DOI] [PubMed] [Google Scholar]
- 38.King KE, Ness PM. How do we prevent transfusion-associated graft-versus-host disease in children? Transfusion. 2011;51:916–20. doi: 10.1111/j.1537-2995.2010.03011.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang-Rodriguez J, Fry E, Fiebig E, et al. Immune response to blood transfusion invery-low-birthweight infants. Transfusion. 2000;40:25–34. doi: 10.1046/j.1537-2995.2000.40010025.x. [DOI] [PubMed] [Google Scholar]
- 40.Hutchinson DL, Turner JH, Schlesinger ER. Persistence of donor cells in neonates after fetal and exchange transfusion. Am J Obstet Gynecol. 1971;109:281–4. doi: 10.1016/0002-9378(71)90876-3. [DOI] [PubMed] [Google Scholar]
- 41.Vietor HE, Hallensleben E, van Bree SP, et al. Survival of donor cells 25 years after intrauterine transfusion. Blood. 2000;95:2709–14. [PubMed] [Google Scholar]
- 42.Marshall CS, Zwerdling T, Dwyre DM, et al. Transfusion associated microchimerism, Hemoglobinopathy, and Autoimmune Disease. Transfusion. 2007;47:110–11A. [Google Scholar]
- 43.Faist E, Schinkel C, Zimmer S. Update on the mechanisms of immune suppression of injury and immune modulation. World J Surg. 1996;20:454–9. doi: 10.1007/s002689900071. [DOI] [PubMed] [Google Scholar]
- 44.Lee TH, Chafets DM, Reed W, et al. Enhanced ascertainment of microchimerism with real-time quantitative polymerase chain reaction amplification of insertion-deletion polymorphisms. Transfusion. 2006;46:1870–8. doi: 10.1111/j.1537-2995.2006.00992.x. [DOI] [PubMed] [Google Scholar]