Abstract
Sixteen new anilide derivatives of the natural trioxane artemisinin were prepared and evaluated for antimalarial efficacy in Plasmodium berghei-infected mice. Of these sixteen new anilides administered orally as one 6 mg/kg dose combined with 18 mg/kg mefloquine hydrochloride, only sulfide 3-arteSanilide 12d was completely curative: on day 30 after infection, all mice in this group had no detectable parasitemia, gained as much weight as the uninfected control mice, and behaved normally.
Keywords: Antimalarial thioethers, sulfoxides, and sulfones; Artemisinin-derived trioxanes; Trioxane monomers SAR
Introduction
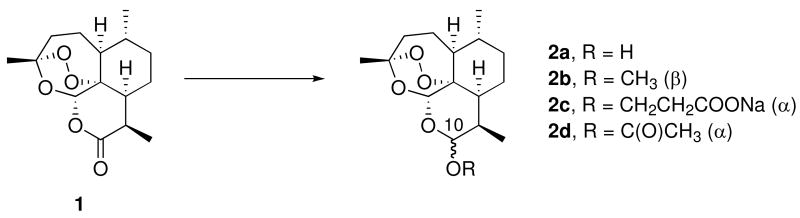
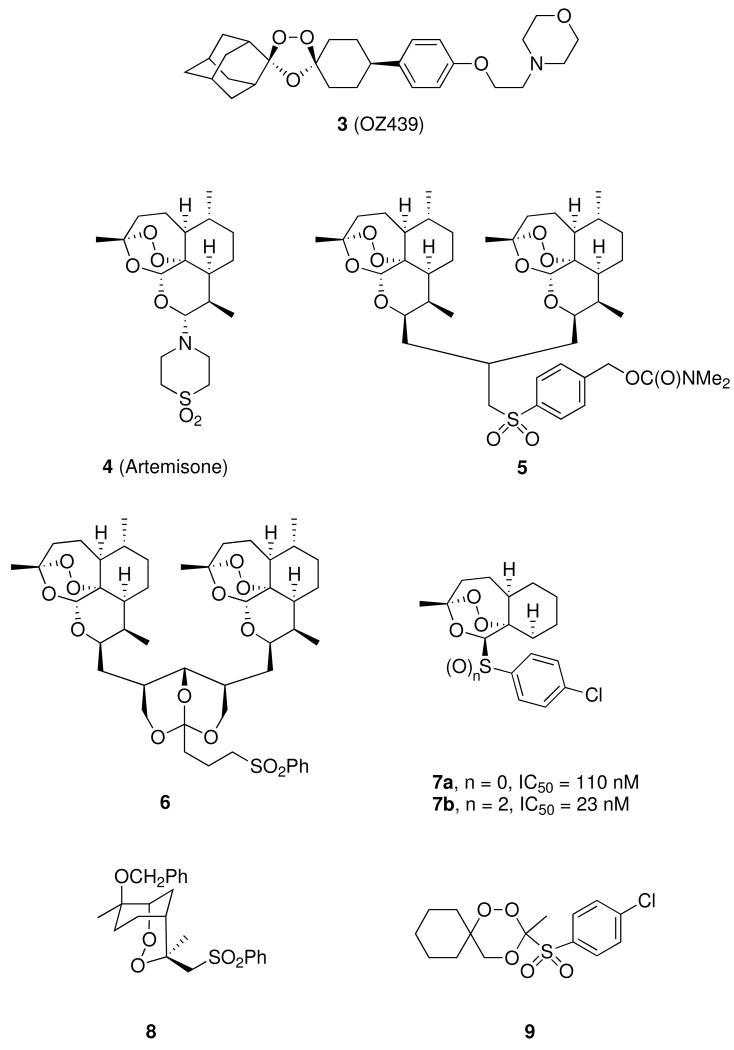
Malaria parasites have developed widespread resistance to standard antimalarial drugs such as chloroquine.1 Therefore, use of non-alkaloidal 1,2,4-trioxanes such as the natural product artemisinin (qinghaosu, 1, Figure 1), combined with a standard alkaloidal antimalarial drug, is now recommended by the World Health Organization (WHO);2 this type of artemisinin combination therapy (ACT) features very rapid parasite clearance by the trioxane as well as prolonged antimalarial activity by the alkaloid, each with a different mechanism of action.3-7 One current ACT drug features a 3-day, 6-dose adult regimen totaling approximately 480 mg of artemether (2b) and 2880 mg of the amino-alcohol lumefantrine.8 Another current ACT drug features a 3-day, 3-dose adult regimen totaling approximately 600 mg of sodium artesunate (2c) and 750 mg of the quinoline mefloquine.9 However, patient compliance with adhering to a repeated dose regimen is often problematic. A recent study reports a 2-day treatment of dihydroartemisinin – piperaquine phosphate – trimethoprim, which reported better patient compliance than the artemether-lumefantrine combination.10 Therefore, a single dose oral cure is highly desirable. A recent report features a single dose oral cure of P. berghei malaria-infected mice using synthetic 1,2,4-trioxolane ozonide OZ439 (3, Figure 2).11 We have recently reported single dose oral cures of P. berghei-infected mice using trioxane dimer sulfone carbamate 5 (Figure 2),12 using dimer orthoester sulfone 6,13 and using trioxane monomer 4-fluoroanilide 12a.14 We report here a new series of trioxane monomer anilides carrying one or two sulfide, sulfoxide, or sulfone substituents on the anilide aromatic ring; one of these new trioxane sulfides (12d) fully cured malaria-infected mice using only one 6 mg/kg oral dose combined with 18 mg/kg mefloquine hydrochloride. Strikingly, this is the first example of an antimalarial trioxane sulfide being more efficacious than its corresponding sulfone.
Figure 1.
Figure 2.
Results and Discussion
Chemistry
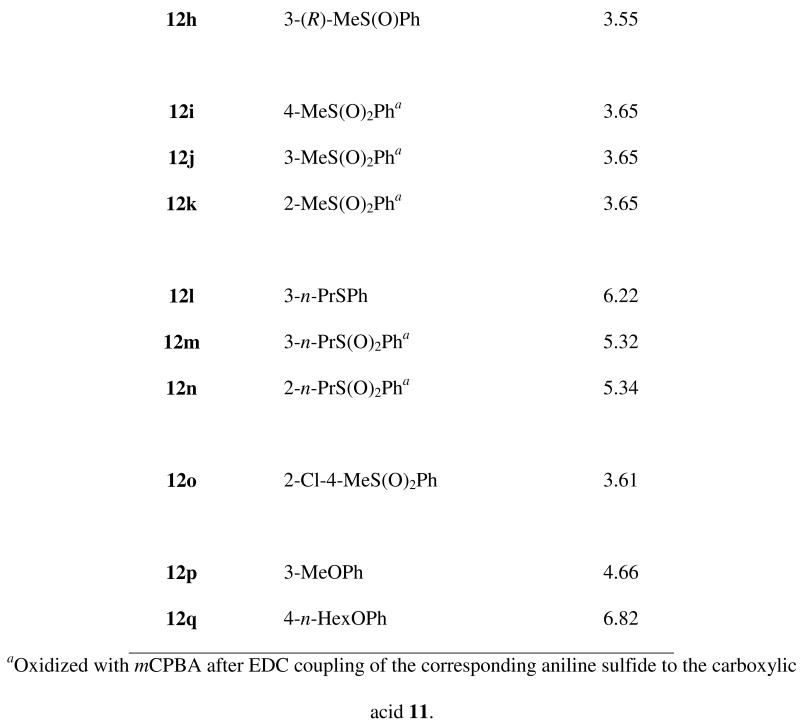
As shown in Scheme 1, artemisinin-derived dihydroartemisinin acetate (2d) reacted with allyl trimethylsilane in the presence of tin tetrachloride to form allyldeoxoartemisinin 10. Hydroboration-oxidation followed by oxidation of the resulting primary alcohol produced C-10 carboxylic acid 11.15 Condensation of carboxylic acid 11 with various anilines yielded a library of trioxane anilides 12b-h, 12l, 12o-q. (Scheme 1). Scale up synthesis is expected to be straightforward. New anilides 12b-q are C-10 non-acetal derivatives; therefore, they are more hydrolytically stabile than the C-10 acetal first generation artemisinin derivatives such as artemether (2b) and artesunate (2c). For example, neat 3-arteSanilide 12d is stable for at least 7 days at 60 °C.
Scheme 1.
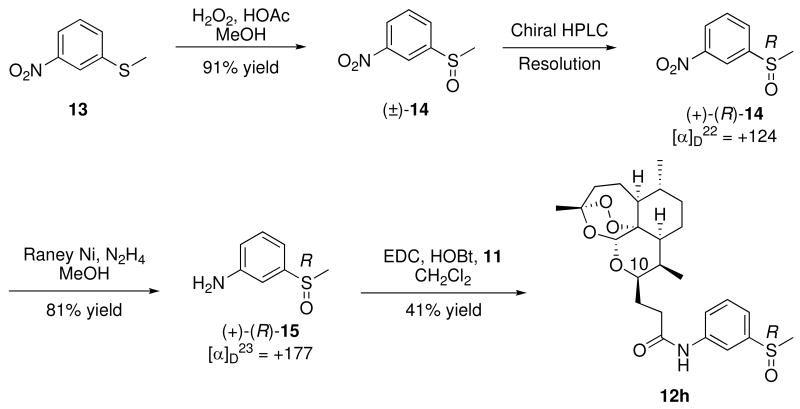
To expand on our SAR, we wanted to probe the oxidation state of the sulfur-containing analogs. Initially, a mixture of sulfoxide diastereomers 12g and 12h was prepared by coupling artemisinin carboxylic acid 11 with racemic amino sulfoxide 15. This diastereomeric mixture was separated by chiral HPLC. Determination of the sulfoxide absolute stereochemistry in anilides 12g and 12h was achieved using enantiomerically pure amino sulfoxide (+)-(R)-15 as follows. Racemic 3-methylsulfinylnitrobenzene (14), prepared by oxidation of the commercially available sulfide 13, was resolved by chiral HPLC; the stereochemistries of the enantiomers were assigned using the specific rotations reported in the literature.16 Reduction of (+)-(R)-3-methylsulfinylnitrobenzene [(+)-(R)-14] with Raney Nickel and hydrazine yielded the enantiomerically pure amino sulfoxide (+)-(R)-15, which was coupled to carboxylic acid 11 to give (R)-sulfoxide diastereomer 12h (Scheme 2). HPLC comparison of the unassigned mixture of sulfoxide diastereomers with the (R)-sulfoxide 12h allowed for the stereochemical sulfoxide assignment to be (S)-12g and (R)-12h. In addition, sulfone anilides 12i-k, 12m, 12n were obtained by oxidation of their corresponding sulfides using meta-chloroperbenzoic acid.
Scheme 2.
Biology – In Vivo Efficacies
Each trioxane (0.64 mg) was combined with mefloquine and dissolved in 0.10 mL of 7:3 Tween 80:ethanol and then diluted with 0.97 mL of deionized water for oral administration to 5-week old, approximately 20 gram C57BL/6J male mice (from the Jackson Laboratory) that were infected intraperitoneally on day 0 with the Plasmodium berghei, ANKA malaria strain (5 × 107 parasitized erythrocytes).12 Each of four mice in a group was treated orally 24 hours post infection with a single dose of 0.20 mL (0.20 mL/1.07 mL × 0.64 mg = 0.12 mg) of diluted trioxane solution, corresponding to a dose of 6 mg/kg of trioxane, combined with 18 mg/kg of mefloquine hydrochloride. Alternatively, a single dose of 7.5 mg/kg of trioxane plus 15.0 mg/kg of mefloquine hydrochloride was used. The malariometrics used involved determining blood parasitemia levels as well as monitoring the duration of animal survival compared to survival time of infected animals receiving no drug.
Three days after infection, an average of 10% blood parasitemia (Giemsa microscopy) was observed in the group of control mice that received no drug, with an average survival time of 6.8 days post infection. The infected mice in this study receiving the trioxane drug artemether (2b) plus mefloquine died an average of 18.8 days post infection (Table 1, controls). In addition, a single oral dose of artemether (6 mg/kg) plus lumefantrine (18 mg/kg) was not curative, with the mice dying an average of 12.5 days post infection. Monotherapy of mefloquine hydrochloride (18 mg/kg single oral dose) prolonged the average survival time of the infected mice to 19.8 days. In comparison, a single oral dose at 18 mg/kg of lumefantrine prolonged mouse survival time to 21.5 days.
Table 1. In Vivo Antimalarial Efficacy Using a Single Oral Dose of Trioxane Combined with Mefloquine Hydrochloride in P. berghei-Infected Mice.
| Single Oral Dose | ||||
|---|---|---|---|---|
| Trioxane | Trioxane (mg/kg) |
Mefloquine Hydrochloride (mg/kg) |
Average Survival (days) after infection | % Suppression of parasitemia (on day 3 post infection) |
| 12b | 6 | 18 | 24.8 (16, 20, 30, 30)a | >99.9 |
| 12c | 7.5 | 15 | 16.3 (15, 15, 16, 19) | >99.9 |
| 12d | 6 | 18 | 30 (30, 30, 30, 30)b | >99.9 |
| 12d | 7.5 | 15 | 30 (30, 30, 30, 30)c | >99.9 |
| 12d | 100 | 0 | 15.0 (7, 7, 16, 30) | 97.2 |
| 12e | 6 | 18 | 27.0 (18, 30, 30, 30) | >99.9 |
| 12f | 6 | 18 | 30 (30, 30, 30, 30)d | >99.9 |
| 12f | 100 | 0 | 7.5 (7,7,8,8) | 97.9 |
| 12g | 6 | 18 | 23.0 (16, 19, 28, 29) | >99.9 |
| 12h | 6 | 18 | 30 (30, 30, 30, 30)e | >99.9 |
| 12i | 7.5 | 15 | 22.5(15, 16, 29, 30) | >99.9 |
| 12j | 7.5 | 15 | 15.5 (15, 15, 16, 16) | 99.9 |
| 12k | 7.5 | 15 | 22.8 (15, 16, 30, 30) | >99.9 |
| 12l | 6 | 18 | 23.0 (15, 18, 29, 30) | >99.9 |
| 12m | 6 | 18 | 11.0 (9, 11, 12, 12) | >99.9 |
| 12n | 6 | 18 | 21.8 (18, 18, 21, 30) | >99.9 |
| 12o | 7.5 | 15 | 24.5 (16, 21, 21, 30) | >99.9 |
| 12p | 6 | 18 | 21.8 (18, 18, 21, 30) | >99.9 |
| 12q | 7.5 | 15 | 15.0 (14, 15, 15, 16) | >99.9 |
|
| ||||
| Controls | ||||
| Vehicle (no drug) | 0 | 0 | 6.8 (6, 7, 7, 7) | 0 |
| Artemether (2b) | 6 | 18 | 18.8 (13, 16, 20, 26) | >99.9 |
| Artemether (2b) | 7.5 | 15 | 19.8 (15, 21, 21, 22) | >99.9 |
| Artemether (2b) | 6 | 18 (lumefantrine) | 12.5 (12, 12, 13, 13) | >99.9 |
| Mefloquine | 0 | 15 | 15.5 (14, 15, 15, 18) | >99.9 |
| Mefloquine | 0 | 18 | 19.8 (16, 16, 20, 27) | >99.9 |
| Lumefantrine | 0 | 18 | 21.5 (12, 22, 25, 27) | >99.9 |
One of the two surviving mice on day 30 post infection had 2% parasitemia.
No parasitemia detected on day 30 post infection.
The four surviving mice had 25-50% parasitemia on day 30 post infection.
Three mice were parasite-free on day 30 but one mouse had 5% parasitemia on day 30 post infection.
One mouse had 1.8% parasitemia on day 30 post infection.
A widely accepted indication of a complete cure (i.e. 100% efficacy) is survival of the mice to day 30 post infection with no detectable malaria parasites in the animals' blood at that time. It is important to note that the combination of the standard trioxane drug artemether (2b), with either mefloquine hydrochloride or lumefantrine, was not curative (Table 1, controls). The average survival times of P. berghei-infected mice receiving a single, oral trioxane dose are shown in Table 1. Important conclusions emerge from these data. While 3-fluoro anilide 12b was not curative, administration of 3-methylthioether 3-arteSanilide 12d at a dose of 7.5 mg/kg plus 15 mg/kg of mefloquine hydrochloride achieved mouse survival through day 30 post infection; however, all four of the surviving mice appeared sick and had considerable parasitemia levels (25-50%). Modification of the dose to 6 mg/kg of 3-arteSanilide 12d and 18 mg/kg of mefloquine resulted in a complete cure, with all mice in this group having gained as much weight by day 30 post infection (data not shown) as the uninfected control mice. In addition, 3-arteSanilide 12d is more efficacious than 4-arteSanilide 12c. From these data, the significance of thioether substitution at the 3-position of the phenyl ring emerged. Bis-sulfide 3,5-arteSSanilide 12f is partially curative at a single oral dose with all four mice alive on day 30 post infection but with one of the four mice possessing 5% parasitemia. Administration of 3-arteSanilide 12d and 3,5-arteSSanilide 12f at non-toxic single oral doses of 100 mg/kg (no mefloquine) resulted in prolonged mouse survival of the 3-arteSanilide 12d dosed mice, compared to essentially no increase in mouse longevity of bis-sulfide 3,5-arteSSanilide 12f treated mice. Replacing the sulfur atom in 12d with an oxygen atom afforded methyl ether 12p, which prolonged survival time to only 21.8 days post infection. This proved the critical nature of the sulfur atom. In addition, the lipophilicity of 3-arteSanilide 12d was increased by lengthening the alkyl sulfide chain from methyl to n-propyl. 3-n-Propyl sulfide 12l, however, is much less efficacious than the curative methyl sulfide 3-arteSanilide 12d.
We also proved the effect of oxidation states of the sulfur atom on antimalarial efficacy. Several sulfide- and sulfone-containing antimalarial trioxanes have been reported in recent literature. For example, artemisone (4, Figure 2), a semi-synthetic trioxane monomer sulfone, is currently in antimalarial clinical trials.17 Trioxane dimer sulfone 512 and trioxane dimer orthoester sulfone 613 cure P. berghei-infected mice, while synthetic trioxane monomer sulfone 7b is at least 4-times more antimalarially potent in vitro than the corresponding sulfide 7a.18 Synthetic sulfonyl endoperoxide 8 is strongly efficacious via oral administration in curing P. berghei-infected mice,19 and synthetic 1,2,4-trioxane sulfone 9 is more active in mice via oral administration than the corresponding sulfide.20,21 Therefore, it was surprising to find that trioxane sulfide 3-arteSanilide 12d, combined with mefloquine, cures malaria-infected mice but that the corresponding sulfone 12j does not (Table 1). In addition, 3-sulfoxide anilide trioxane diastereomers 12g and 12h have different antimalarial activities. 3-(R)-Sulfoxide 12h is partially curative and possesses similar antimalarial efficacy to that of 3-arteSanilide 12d. In contrast, the diastereomeric 3-(S)-sulfoxide 12g prolongs the average animal life span to only 23.0 days.
As further evidence of the complete cure of malaria-infected mice achieved by a single 6 mg/kg dose oral dose of 3-arteSanilide 12d plus 18mg/kg mefloquine, blood from the cured mice in this group was inoculated into uninfected mice; no parasitemia was detected in the inoculated mice after 30 days. 22
Biology – In Vitro Potencies
Prompted by the unexpected in vivo efficacy of 3-arteSanilide 12d, we assayed in vitro the intrinsic antimalarial activity, free of host-mediated factors, of compounds that differ in the oxidative state of the sulfur atom (Table 2). In keeping with the rodent study, sulfide 12d is more potent than (S)-sulfoxide 12g or sulfone 12j.
Table 2. In Vitro Antimalarial Potencies of Trioxanes against P. falciparum (NF54) Parasites.
| Trioxane | Antimalarial Activitya EC50, nM |
|---|---|
| 12d | 9.1 ± 0.57 |
| 12f | 6.5 ± 0.28 |
| 12g | 23 ± 1.3 |
| 12h | 29 ± 0.43 |
| 12j | 21 ± 1.1 |
| Control | |
| Artemisinin (1) | 10 ± 1.1 |
Values are M ± SD of at least four determinations; artemisinin activity is for concurrent controls.
Conclusion
P. berghei-infected mice receiving 3-arteSanilide 12d were not only completely cured but also gained as much weight as the uninfected control mice. Furthermore, neither overt toxicity nor behavioral change attributable to trioxane administration was observed in any of the malaria-infected mice cured by 3-arteSanilide 12d combined with mefloquine hydrochloride.
Experimental Section
1H-NMR (400 or 300 MHz), 13C-NMR (100 or 75 MHz), and 19F-NMR (282 MHz) spectra were recorded on Bruker spectrometer using the residual solvent peak or trichlorofluoromethane as an internal standard. High resolution mass spectrum-fast atom bombardment (HRMSFAB) were obtained using a VG70SE double focusing magnetic sector mass spectrometer (VG Analytical, Manchester, UK nowMicromass/Waters) equipped with a Cs+ ion gun (28 kV(2 μA), an off-axis multiplier and a MSS data system (MasCom, Bremen,Germany). The resolution of the instrument was set at 10000 (100 ppm peak width). Samples were mixed with m-nitrobenzyl alcohol matrix deposited on the target of a direct insertion probe for introduction into the source. For accurate mass measurements, a mass scan range was employed with the matrix containing 10% polyethylene glycol (PEG) or polyethylene glycol, monomethyl ether (PEGMME) mass calibrant. Low resolution mass spectra (electrospray ionization) were acquired on an Agilent Technologies 6130 quadrupole spectrometer coupled to an Agilent Technologies 1200 series HPLC. High resolution mass spectrum-electron ionization sprary (HRMS-ESI) were obtained on an Agilent Technologies 1200 series Dual Absorbance Detector HPLC system equipped with a Phenomenex Luna 75×3mm, C18, 3μm column at 45°C (UV detection at 220nm, BW 8nm, and 254nm BW 8nm, flow rate: 0.8 mL/min (increasing), Injection volume: 1.0 μL, sample solvent: 100% Methanol, sample conc.: ∼0.01 mg/mL, mobile phase A: Water with 0.1% acetic acid, mobile phase B: Acetonitrile with 0.1% acetic acid) coupled to a Agilent 6210 time-of- flight mass spectrometer (ion source: Duel ESI, min range: 115 m/z, max range: 1400 m/z, scan rate: 0.9 seconds, gas temp: 340°C, gas flow: 10 L/min, nebulizer: 50 PSI, ion polarity: positive, VCap: 3500 V, fragmentor: 175 V, skimmer1: 65 V, OctopoleRFPeak: 250 V, ref mass: enabled (Agilent P/N G1969-85001). Data were analyzed using Agilent Masshunter Workstation Data Acquisition (v B.02.00, Patch 1,2,3) and Agilent Masshunter Qualitative Analysis (v B.02.00, Build 2.0.197.7, Patch 3). Fourier transform-infrared (FT-IR) experiments were performed on a Bruker Vector 22 instrument. Optical rotation values were obtained using a 100 mm quartz cell on a JASCO P-1010 polarimeter with a 589 nm source. The purity of analogs 12b-12q was determined to be >95% by HPLC. HPLC data were acquired using a Varian ProStar 210 two pump system with a ProStar 325 dual wavelength detector set at 215 nm and 254 nm. Chiral Columns ((S,S)-Whelk-0 5/100 Kromasil 25cm × 4.6 cm I.D. and RegisCell 25cm × 4.6cm ID) were purchased from Regis Technologies. Log P values were calculated by using MarvinSketch and a calculator plug-in by ChemAxon Kft.
Synthesis of 3-arteSanilide 12d
To an oven dried 10 mL round bottom flask was added carboxylic acid monomer 11 (15 mg, 0.044 mmol), EDC (9.3 mg, 0.048 mmol), HOBt (6.5 mg, 0.048 mmol), and CH2Cl2 (1 mL). The reaction was stirred for 1 hour before commercially available 3-aminothioanisole (6.5 μL, 0.053 mmol) was added dropwise and stirred for an additional 18 hours at room temperature until TLC analysis indicated consumption of starting material. The reaction was quenched with brine (3 mL), and extracted with CH2Cl2 (3 × 3 mL). The resulting organic extracts were dried over MgSO4 and concentrated in vacuo. The crude product was purified by preparative thin layer chromatography (silica gel, 40% ethyl acetate/hexanes) to afford 12d as a colorless, amorphous solid (88% yield, 18.0 mg, 0.039 mmol). FT-IR (thin film, cm-1) 3331, 2941, 1670, 1550, 1466, 1384, 1301, 1299, 1106, 1053; 1H-NMR (400 MHz, CDCl3) δ 7.78 (bs, 1H), 7.57 (s, 1H), 7.25 (d, J = 8.8 Hz, 1H), 7.19 (t, J = 8.0 Hz, 1H), 6.97 (t, J = 7.6 Hz, 1H), 5.35 (s, 1H), 4.17 (m, 1H), 2.77-2.59 (m, 2H), 2.48 (m, 1H), 2.47 (s, 3H), 2.33 (m, 1H) 2.05-1.76 (m, 5H), 1.62 (m, 2H), 1.49-1.22 (m, 5H), 1.39 (s, 3H), 0.95 (d, J = 5.6 Hz, 3H), 0.89 (d, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) 171.4, 139.3, 138.7, 129.0, 122.0, 117.3, 116.3, 103.4, 88.9, 81.1, 76.0, 52.3, 44.4, 37.4, 36.5, 36.0, 34.4, 30.2, 26.1, 26.1, 24.9, 24.6, 20.2, 15.6, 13.1. [a]D26 = +51.3 (c = 0.72, CHCl3). HRMS m/z for C25H36NO6(M+H)+ calculated, 463.2392 found 463.2390.
Synthesis of 3,5-ArteSSanilide 12f
To an oven dried 10 mL round bottom flask was added carboxylic acid monomer 11 (15 mg, 0.044 mmol), EDC (9.3 mg, 0.048 mmol), HOBt (6.5 mg, 0.048 mmol), and 3,5-bis(methylsulfanyl)aniline (9.8 mg, 0.053 mmol). The contents were dissolved in CH2Cl2 (1 mL) and stirred for 18 hours at room temperature until TLC analysis indicated consumption of starting material. The reaction was quenched with brine (3 mL), and extracted with CH2Cl2 (3 × 3 mL). The resulting organic extracts were dried over MgSO4 and concentrated in vacuo. The crude product was purified by preparative thin layer chromatography (silica gel, 40% ethyl acetate/hexanes) to afford 12d as a colorless, amorphous solid (61% yield, 13.6 mg, 0.027 mmol). FT-IR (thin film, cm-1) 3333, 2989, 1661, 1541, 1451, 1368, 1289, 1204, 1045, 1008; 1H-NMR (400 MHz, CDCl3) δ 7.71 (bs, 1H), 7.60 (s, 2H), 7.43 (s, 1H), 5.32 (s, 1H), 4.20 (m, 1H), 2.79-2.49 (m, 2H), 2.44 (m, 1H), 2.40 (s, 6H), 2.32 (m, 1H) 2.21-1.70 (m, 4H), 1.59 (m, 3H), 1.42-1.22 (m, 5H), 1.42 (s, 3H), 0.94 (d, J = 6.0 Hz, 3H), 0.90 (d, J = 7.9 Hz, 3H). 13C NMR (100 MHz, CDCl3) 171.0, 138.8, 138.3, 128.0, 121.0, 116.4, 116.1, 100.8, 87.5, 80.2, 75.5, 52.1, 50.4, 47.2, 38.1, 36.6, 365.7, 34.2, 30.7, 26.2, 25.8, 23.1, 22.2, 20.1, 16.0, 12.9. [a]D23 = +43 (c = 0.40, CHCl3). HRMS m/z calculated for C28H36S2NO5 (M + H)+ 508.7136, found 508.7139.
Synthesis of 3-methyl sulfoxides 12g and 12h
Carboxylic acid 11 (15 mg, 0.044 mmol), EDC (9.3 mg, 0.048 mmol), and HOBt (6.5 mg, 0.048 mmol) were dissolved in CH2Cl2 (2 mL) in a 10 mL round bottom flask. The solution was stirred for 1 hour at room temperature before (±)-15 (8.1 mg, 0.053 mmol) was added. The reaction was allowed to stir for 48 hours before it was quenched with brine (3 mL) and extracted with CH2Cl2 (3 × 4 mL). The combined organic layers were dried with MgSO4 and concentrated under reduced pressure. The resulting crude oil was purified by preparative thin layer chromatography (silica gel, 100% EtOAc) to afford a 1:1 diastereomeric mixture of 12g and 12h (51% yield, mg, 10.5 mg, 0.022 mmol). This mixture was separated by HPLC (Regis Whelk-01 (S,S); 10-50% IPA in hexanes; detection wavelength 254 nm; flow rate = 2.5 mL/min) tr = 115.1 min (S)-sulfoxide 12g and 128.1 min (R)-sulfoxide 12h. Spectral data shown below.
Spectral Data of 3-(S)-Sulfoxide 12g
Spectral Data of 3-(S)-Sulfoxide 12g as an amorphous, white solid: 1H-NMR (400 MHz, CDCl3) δ 8.50 (d, J = 8.5 Hz, 1H), 7.82 (dd, J = 7.4, 1.2 Hz, 1H), 7.60 (t, J = 7.2 Hz, 1H), 7.24 (t, J = 7.2 Hz, 1H), 5.32 (s, 1H), 4.20 (dd, J = 9.1, 6.8 Hz, 1 H), 3.10 (m, 2H), 2.75 (s, 3H) 2.73 (m, 2H), 2.51 (m, 1H), 2.31 (td, J = 14.1, 3.6 1H), 2.04 (m, 2H), 1.71 (m, 4H), 1.70 (m, 4H), 1.36 (s, 3H), 0.94 (d, J = 6 Hz, 3H), 0.88 (d, J = 7.5 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.2, 137.3, 134.9, 129.9, 125.8, 123.6, 123.0, 103.7, 89.0, 87.1, 75.4, 57.8, 48.8, 40.0, 36.6, 36.3, 36.1, 34.4, 30.2, 26.1, 24.9, 24.7, 20.2, 16.1, 13.0. [a]D24 = +29 (c = 0.12, CHCl3); [a]D24 = +44 (c = 0.12, CHCl3). HRMS m/z calculated for C25H36SNO6(M+H)+ 478.2263, found 478.2266.
Spectral Data of 3-(R)-Sulfoxide 12h
Spectral Data of 3-(R)-Sulfoxide 12h as an amorphous, white solid: 1H-NMR (300 MHz, CDCl3) δ 8.28 (bs, 1H), 7.88 (m, 1H), 7.75 (d, J = 6.9 Hz, 1H) 7.47 (t, J = 7.8 Hz, 1H), 7.35 (d, J = 7.8 Hz, 1H), 5.35 (s, 1H), 4.22 (m, 1H), 2.79-2.46 (m, 5H), 2.74 (s, 3H), 2.34 (td, J = 14.4, 3.9 Hz, 1H), 2.09-1.79 (m, 5H), 1.69-1.55 (m, 2H), 1.50-1.20 (m, 3H), 1.38 (s, 3H), 0.96 (d, J = 5.7 Hz, 3H), 0.91 (d, J = 7.5 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 172.0, 137.4, 134.9, 129.9, 125.6, 123.3, 122.9, 103.9, 89.3, 87.0, 75.4, 57.2, 48.6, 39.8, 36.8, 36.3, 36.0, 34.5, 30.1, 26.3, 24.8, 24.3, 20.2, 16.1, 13.0; [a]D24 = +61 (c = 0.12, CHCl3). HRMS m/z calculated for C25H36SNO6 (M+H)+ 478.2263, found 478.2265.
Synthesis of 3-sulfone 12j
3-ArteSanilide 12d (16.1 mg, 0.035 mmol) was dissolved in CH2Cl2 (1.5 mL) to which mCPBA (≤77%, 17.1 mg, 0.077 mmol) was added and stirred for 2.5 hours. The reaction was quenched with NaHCO3 (aq, 2 mL) and extracted with CH2Cl2 (3 × 3 mL). The organic layers were washed with saturated NaHCO3 and saturated NaHSO3, dried with MgSO4, concentrated in vacuo, and purified by preparative thin layer chromatography (silica gel, 60%, ethyl acetate/hexanes) to yield 12j as a colorless, amorphous solid (94% yield, 16.2 mg, 0.033 mmol). FT-IR (thin film, cm-1) 3298, 2921, 1666, 1570, 1531, 1444, 1372, 1296, 1124, 1092, 1058, 1008; 1H-NMR (300 MHz, CDCl3) δ 8.32 (bs, 1H), 8.11 (s, 1H), 7.95 (d, J = 8.2 Hz, 1H), 7.65 (d, J = 7.9, 1H), 7.50 (t, J = 8.0 Hz, 1H), 5.36 (s, 1H), 4.19 (m, 1 H), 3.06 (s, 3H), 2.79-2.50 (m, 3H), 2.33 (m, 1H), 2.04-1.58 (m, 6H), 1.46-1.16 (m, 7H), 1.38 (s, 3H), 0.96 (d, J = 9.0 Hz, 3H), 0.83 (d, J = 6.8 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 171.9, 138.6, 135.3, 129.9 128.8, 126.4, 123.7, 122.9, 103.3, 89.0, 87.1, 75.4, 57.8, 54.6, 43.8, 37.4, 36.5, 36.1, 34.4, 30.2, 26.1, 24.9, 24.4, 20.2, 12.9. [α]D22 = +41 (c = 0.19, CHCl3) HRMS m/z calculated for C25H36NO7S(M+H)+ 494.2212, found 494.2216.
Supplementary Material
Acknowledgments
We thank the NIH (AI 34885 to G.H.P. and RR025005 to T.A.S.), the Johns Hopkins Malaria Research Institute, and the Bloomberg Family Foundation for financial support (to G.H.P. and T.A.S.).
Abbreviations
- ACT
artemisinin combination therapy
- EDC
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
- HOBT
1-hydroxybenzotriazole
- DMSO
dimethyl sulfoxide
- mCPBA
meta-chloroperbenzoic acid
Footnotes
Supporting Information Available: Spectral data for compounds 12b, 12c, 12e, 12i, 12k-12q. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Olliaro PL, Boland PB. Clinical Public Health Implications of Antimalarial Drug Resistance. In: Rosenthal PJ, editor. Antimalarial Chemotherapy: Mechanisms of Action, Resistance, and New Directions in Drug Discovery. Humana Press; Totowa, NJ: 2001. pp. 65–83. [Google Scholar]
- 2.Guidelines for the Treatment of Malaria. World Health Organization; Geneva: 2006. [Google Scholar]
- 3.Ashley EA, White NJ. Artemisinin-based Combinations. Curr Opin Infect Dis. 2005;18:531–536. doi: 10.1097/01.qco.0000186848.46417.6c. [DOI] [PubMed] [Google Scholar]
- 4.(a) Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N. Artesunate Combinations for Treatment of Malaria: Meta-analysis. Lancet. 2004;363:9–17. doi: 10.1016/s0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]; (b) Guthmann JP, Cohuet S, Rigutto C, Fortes F, Saraiva N, Kiguli J, Kyomuhendo J, Francis M, Noel F, Mulemba M, Balkan S. High Efficacy of Two Artemisinin-based Combinations (Artesunate plus Amodiaquine and Artemether plus Lumefantrine) in Caala, Central Angola. Am J Trop Med Hyg. 2006;75:143–145. [PubMed] [Google Scholar]
- 5.Myint HY, Ashley EA, Day NPJ, Nosten F, White NJ. Efficacy and Safety of Dihydroartemisinin-piperaquine. Trans R Soc Trop Med Hyg. 2007;101:858–866. doi: 10.1016/j.trstmh.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Sirima SB, Tiono AB, Gansane A, Diarra A, Ouedraogo A, Konate AT, Kiechel JR, Morgan CC, Olliaro PL, Taylor WRJ. The Efficacy and Safety of a New Fixed-dose Combination of Amodiaquine and Artesunate in Young African Children with Acute Uncomplicated. Plasmodium falciparum Malar J. 2009;8:48. doi: 10.1186/1475-2875-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Pilla Varotti F, Botelho ACC, Andrade AA, de Paula RC, Fagundes EMS, Valverde A, Mayer LMU, Mendonca JS, de Souza MVN, Boechat N, Krettli AU. Synthesis, Antimalarial Activity, and Intracellular Targets of MEFAS, a New Hybrid Compound Derived from Mefloquine and Artesunate. Antimicrob Agents Chemother. 2008;52:3868–3874. doi: 10.1128/AAC.00510-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagara I, Diallo AD, Kone M, Coulibaly M, Diawara SI, Guindo O, Maiga H, Niambele MB, Sissoko M, Dicko A, Dj2imde A, Doumbo OK. A Randomized Trial of Artesunate-mefloquine versus Artemether-lumefantrine for Treatment of Uncomplicated Plasmodium falciparum Malaria in Mali. Am J Trop Med Hyg. 2008;79:655–661. [PubMed] [Google Scholar]
- 9.Bhatt KM, Samia BM, Bhatt SM, Wasunna KM. Efficacy and Safety of an Artesunate/mefloquine Combination, (Artequin) in the Treatment of Uncomplicated P. falciparum Malaria in Kenya. East Afr Med J. 2006;83:236–242. doi: 10.4314/eamj.v83i5.9428. [DOI] [PubMed] [Google Scholar]
- 10.Menan H, Faye O, Same-Ekobo A, Oga ASS, Faye B, Barro CPK, Kuete T, N'diaye JL, Vicky AH, Tine R, Yavo W, Kane D, Kassi KF, Kone M. Comparative study of dihydroartemisinin-piperaquine phosphate – trimethoprim versus artemether – lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Cameroon, Ivory Coast and Senegal. Malaria Journal. 2011;10:185–193. doi: 10.1186/1475-2875-10-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charman SA, Arbe-Barnes S, Bathurst IC, Brun R, Campbell M, Charman WN, Chiu FCK, Chollet J, Craft JC, Creek DJ, Dong Y, Matile H, Maurer M, Morizzi J, Nguyen T, Papastogiannidis P, Scheurer C, Shackleford DM, Sriraghavan K, Stingelin L, Tang Y, Urwyler H, Wang X, White KL, Wittlin S, Zhou L, Vennerstrom JL. Synthetic Ozonide Drug Candidate OZ439 Offers New Hope for a Single-dose Cure of Uncomplicated Malaria. PNAS. 2011;108:4400–4405. doi: 10.1073/pnas.1015762108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenthal AS, Chen X, Liu JO, West DC, Hergenrother PJ, Shapiro TA, Posner GH. Malaria-infected Mice are Cured by a Single Oral Dose of New Dimeric Trioxane Sulfones Which are Also Selectively and Powerfully Cytotoxic to Cancer Cells. J Med Chem. 2009;52:1198–1203. doi: 10.1021/jm801484v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon DK, Tripathi A, Sullivan D, Siegler MA, Parkin S, Posner GH. A Single, Low, Oral Dose of a 5-Carbon-linked Trioxane Dimer Orthoester Plus Mefloquine Cures Malaria-infected Mice. Bioorg Med Chem Lett. 2011;21:2773–2775. doi: 10.1016/j.bmcl.2010.09.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodard LE, Chang W, Chen X, Liu JO, Shapiro TA, Posner GH. Malaria-Infected Mice Live Until at Least Day 30 After a New Monomeric Trioxane Combined with Mefloquine are Administered Together in a Single Low Oral Dose. J Med Chem. 2009;52:7458–7462. doi: 10.1021/jm9005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posner GH, Paik IH, Sur S, McRiner AJ, Borstnik K, Xie S, Shapiro TA. Orally Active, Antimalarial, Anticancer, Artemisinin-derived Trioxane Dimers with High Stability and Efficacy. J Med Chem. 2003;46:1060–1065. doi: 10.1021/jm020461q. [DOI] [PubMed] [Google Scholar]
- 16.Tohma H, Takizawa S, Watanabe H, Fukuoka Y, Maegawa T, Kita Y. Hypervalent Iodine(V)-Induced Asymmetric Oxidation of Sulfides to Sulfoxides Mediated by Reversed Micelles: Novel Nonmetallic Catalytic System. J Org Chem. 1999;64:3519–3523. doi: 10.1021/jo982295t. [DOI] [PubMed] [Google Scholar]
- 17.Haynes RK, Fugmann B, Stetter J, Rieckmann K, Heilmann HD, Chan HW, Cheung MK, Lam WL, Wong HN, Croft SL, Vivas L, Rattray L, Stewart L, Peters W, Robinson BL, Edstein MD, Kotecka B, Kyle DE, Beckermann B, Gerisch M, Radtke M, Schmuck G, Steinke W, Wollborn U, Schmeer K, Römer A. Artemisone–A Highly Active Antimalarial Drug of the Artemisinin Class. Angew Chem Int Ed. 2006;45:2082–2088. doi: 10.1002/anie.200503071. [DOI] [PubMed] [Google Scholar]
- 18.Posner GH, O'Dowd H, Caferro T, Cumming JN, Ploypradith P, Xie S, Shapiro TA. Antimalarial Synthetic Sulfone Trioxanes. Tetrahedron Lett. 1998;39:2273–2276. [Google Scholar]
- 19.Bachi MD, Korshin EE, Hoos R, Szpilman AM, Ploypradith P, Xie S, Shapiro TA, Posner GH. A Short Synthesis and Biological Evaluation of Potent and Nontoxic Antimalarial Bridged Bicyclic β-Sulfonyl-Endoperoxides. J Med Chem. 2003;46:2516–2533. doi: 10.1021/jm020584a. [DOI] [PubMed] [Google Scholar]
- 20.Amewu R, Gibbon P, Mukhtar A, Stachulski AV, Ward SA, Hall C, Rimmer K, Davies J, Vivas L, Bacsa J, Mercer AE, Nixon G, Stocks PA, O'Neill PM. Synthesis, in vitro and in vivo Antimalarial Assessment of Sulfide, Sulfone and Vinyl Amide-substituted 1,2,4-Trioxanes Prepared via Thiol-olefin Co-oxygenation (TOCO) of Allylic Alcohols. Org Biomol Chem. 2010;8:2068–2077. doi: 10.1039/b924319d. [DOI] [PubMed] [Google Scholar]
- 21.Jung M, Tak J, Chung WY, Park KK. Antiangiogenic Activity of Deoxoartemisinin Derivatives on Chorioallantoic Membrane. Bioorg Med Chem Lett. 2006;16:1227–1230. doi: 10.1016/j.bmcl.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 22.Burrows NJ, Chibale K, Wells TNC. The State of the Art in Anti-Malarial Drug Discovery and Development. Curr Top Med Chem. 2011;11:1226–1254. doi: 10.2174/156802611795429194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.