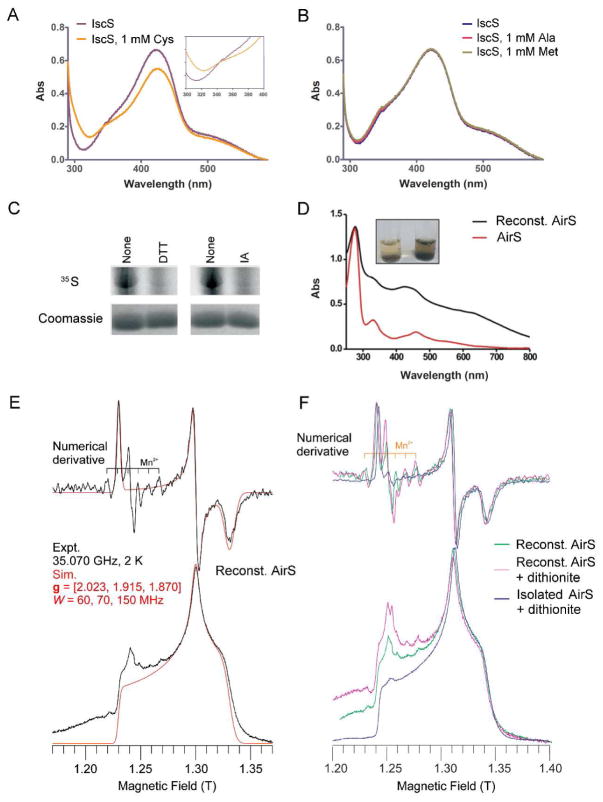
Figure 2.
S. aureus IscS (SA1450) mediates AirS Fe-S cluster reconstitution. (A and B) UV-Vis absorbance spectra of S. aureus IscS showing a major peak at 420 nm. Spectroscopic analysis was performed in a 0.1-ml cuvette containing purified S. aureus IscS (30 μM) before and after adding L-Cys or L-Ala or L-Met. (Inset) Perturbation of the spectrum of IscS upon addition of L-Cys. (C) Effect of reducing (DTT) and alkylating (iodoacetamide, IA) agents on the IscS-based sulfur transfer reaction. The reaction was carried out at room temperature using 35S-labeled L-Cys and IscS (lane 1), modification of IscS with 10 mM DTT (left panel), and modification with 1 mM iodoacetamide (right panel). All reactions were analyzed by 13% SDS-PAGE followed by autoradiography. Coomassie blue staining was used to ensure the quality of the loaded protein. (D) Absorbance spectra of AirS before and after IscS reconstitution. Reconstitution was performed in an anaerobic chamber described in Experimental Methods. (Inset) Development of a green-brown color before and after IscS reconstitution. (E) Q-band EPR spectrum of the reconstituted AirS. The g values are 2.023, 1.915, and 1.870. (F) Overlay of Q-band EPR spectra of AirS directly isolated from E. coli after dithionite reduction (g = [2.023, 1.915, 1.870]) and reconstituted AirS before and after dithionite reduction (g = [2.023, 1.915, 1.870]). Very weak signals from exogenous Mn2+ and a radical (both centered at g = 2.00) are also seen (E and F). Mn2+ is a minor contaminant in the Fe2+ salts used for cluster reconstitution; the origin of the radical is unknown, but may be a consequence of dithionite reduction.