SUMMARY
Mice with targeted deletion of the growth hormone receptor (GHRKO mice) are GH resistant, small, obese, hypoinsulinemic, highly insulin sensitive and remarkably long-lived. To elucidate the unexpected coexistence of adiposity with improved insulin sensitivity and extended longevity, we examined effects of surgical removal of visceral (epididymal and perinephric) fat on metabolic traits related to insulin signaling and longevity. Comparison of results obtained in GHRKO mice and in normal animals from the same strain revealed disparate effects of visceral fat removal (VFR) on insulin and glucose tolerance, adiponectin levels, accumulation of ectopic fat, phosphorylation of insulin signaling intermediates, body temperature and respiratory quotient (RQ). Overall, VFR produced the expected improvements in insulin sensitivity and reduced body temperature and RQ in normal mice and had opposite effects in GHRKO mice. Some of the examined parameters were altered by VFR in opposite directions in GHRKO and normal mice, others were affected in only one genotype or exhibited significant genotype × treatment interactions. Functional differences between visceral fat of GHRKO and normal mice were confirmed by measurements of adipokine secretion, lipolysis and expression of genes related to fat metabolism. We conclude that in the absence of GH signaling the secretory activity of visceral fat is profoundly altered and unexpectedly promotes enhanced insulin sensitivity. The apparent beneficial effects of visceral fat in GHRKO mice may also explain why reducing adiposity by calorie restriction fails to improve insulin signaling or further extend longevity in these animals.
Keywords: GHRKO, insulin, adipose tissue
INTRODUCTION
Studies of longevity and aging in species as varied as the microscopic worm Caenoerhabditis elegans, the fly Drosophila melanogaster, and the mouse Mus musculus indicate that partial suppression of insulin/insulin like growth factor-1 (IGF-1) signaling pathway positively correlates with extended longevity (1–5). Loss of function mutations of the Prop1 or Pit1 genes which produce deficiency of growth hormone (GH), prolactin (PRL) and thyrotropin in Ames and Snell dwarf mice or elimination of the GH receptor gene in GH receptor/GH binding protein knockout (GHRKO) mice cause precipitous decline in circulating IGF-1, significant life extension and increased insulin sensitivity (1;3–7).
Calorie restriction (CR) extends longevity in mammals (5;8), at least partially, by altering the insulin/IGF-1 signaling pathway (5;8–13). In Ames dwarf mice, CR further extends lifespan and enhances insulin sensitivity (1), suggesting actions via similar although not identical pathways. Surprisingly, the same dietary intervention altered neither insulin sensitivity nor longevity in the GHRKO mouse (6). One could therefore speculate that GHR knockout and CR might act via the same mechanisms. One of the consistently observed actions of CR is a decrease in the amount of adipose tissue, and there is considerable evidence that the amount of fat tissue is positively associated with insulin resistance. However, in sharp contrast to CR animals, GHRKO mice are obese in comparison to their normal siblings (14;15), yet are insulin sensitive, healthy and long-lived (4;6). It has been demonstrated that the site of fat accumulation may be more critical for health than the overall amount of fat tissue. Visceral (intra-abdominal or “central”) obesity promotes insulin resistance, and increases the risk of type 2 diabetes, dyslipidemia and mortality (16–19). Peripheral obesity—i.e., the increased amount of subcutaneous fat—is associated with increased insulin sensitivity and lower risk of type 2 diabetes and dyslipidemia (20–22). In humans omentectomy (removal of visceral fat) was shown to reduce insulin and glucose levels (23), whereas liposuction targets only subcutaneous fat tissue and does not result in any improvement of metabolic parameters (24). Diet and exercise result in preferential loss of visceral rather than subcutaneous fat and promote improvement of metabolic parameters (25). In rodents, fat distribution is also important in regulating insulin signaling. Mice subjected to a high fat diet (HFD) are characterized by increased visceral fat accumulation with a parallel decrease of insulin sensitivity (26–28). Aging is typically accompanied by an increase of visceral fat and decrease in insulin sensitivity, while surgical visceral fat removal (VFR) in rats improved insulin sensitivity and, more importantly, increased longevity (26;29).
It is possible that obesity in the GHRKO mouse (14) represents a form of “healthy” obesity, because it involves preferential accumulation of subcutaneous fat although visceral fat may also be increased. Different, and conceivably beneficial, functions of visceral fat in GHRKO mice could also explain many of their phenotypic characteristics as well as lack of responses of these animals to CR in terms of insulin signaling and longevity (6;30). The present study was undertaken to compare the responses to VFR in GHRKO and normal mice. We hypothesized that the benefits of this intervention previously shown in genetically normal animals will be absent or reversed in the GHRKO animals.
RESULTS
CHARACTERISTICS OF ADIPOSE TISSUE
Epididymal and perinephric fat adipocytokines levels
The multiplex cytokines analysis indicated that IL-6 was downregulated in both epididymal and perinephric GHRKO fat pads when compared to the same fat pads from normal animals (P<0.024 and P<0.044, respectively) (Table 1). Resistin level was decreased in perinephric (P<0.036) but not epididymal fat from GHRKO mice when compared to the same fat depots from normal mice (Table 1). There were no significant differences in the levels of MCP-1, TNFα, leptin or PAI-1 (Table 1).
Table 1.
The levels of resistin, leptin, TNFα, IL-6, PAI-1 and MCP-1 in epididymal and perinephric fat in growth hormone receptor/binding protein knockout (GHRKO) and normal mice (n=8–10). Means ± SEM.
| Normal Epididymal |
GHRKO Epididimal |
Normal Perinephric |
GHRKO Perinephric |
|
|---|---|---|---|---|
| Resistin | 52638 ±8756a,c | 38075 ±1889a | 88566 ±7911b | 68309 ±5713c |
| Leptin | 14393 ±3131a,b | 9476 ±1777a | 182888 ±3199b | 12698 ±3287a,b |
| TNFα | 3.477 ±0.659a | 2.589 ±0.240a | 5.306 ±0.580a | 4.456 ±0.230a |
| IL-6 | 12.3 ±1.3a | 8.9 ±0.48b | 67.6 ±10.5c | 31.2 ±5.9d |
| PAI-1 | 214.9 ±42.7a | 187.4 ±32a | 249.99 ±29.7a | 213.5 ±39a |
| MCP-1 | 155.4 ±57a,b | 92.9 ±27.3a | 133.2 ±45a,b | 259 ±43b |
values that do not share the same letter in the superscript are statistically significant (P<0.05).
Gene expression in epididymal, perinephric and subcutaneous fat
Using real-time PCR the analysis of genes involved in lipid metabolism was performed in epididymal, perinephric and subcutaneous fat depots. Expression of six of the nine analyzed genes was increased in epididymal fat from GHRKO mice when compared to epididymal fat from normal controls. These genes included insulin receptor (IR) (P<0.031), peroxisome proliferator activated receptor gamma (PPARγ) (p<0.0148), PPARα (P<0.0314), PPARγ coactivator 1 (PGC1α) (P<0.047), sterol regulatory element binding proteins (SERBPs) (P<0.05), and hormone sensitive lipase (HSL) (P<0.031). The uncoupling protein 2 (UCP2) was increased in perinephric fat from GHRKO animals when compared to normal mice (P<0.039). No significant differences between GHRKO and normal mice were detected in the expression of other genes in the perinephric fat pads or any of the examined genes in the subcutaneous fat (Table 2).
Table 2.
The levels of relative expression of genes related to lipid metabolism in epididymal, perinephric and subcutaneous fat tissues from GHRKO and normal mice (n=8–10). Means ± SEM.
| Genes | Epididymal fat | Perinephric fat | Subcutaneous fat | |||
|---|---|---|---|---|---|---|
| Normal | GHRKO | Normal | GHRKO | Normal | GHRKO | |
| IR | 1±0.29 | 9.5±3.8 * | 1.65±0.85 | 3.2±1 NS | 0.5±0.14 | 1.43±1 NS |
| GLUT4 | 1±0.46 | 12.6±5.9NS | 0.75±0.25 | 1.6±0.69NS | 0.58±0.35 | 3.1±2.3 NS |
| PPARγ | 1±0.13 | 1.6±0.16* | 0.58±0.14 | 0.7±0.16NS | 0.497±0.13 | 0.74±0.2NS |
| PPARα | 1±0.24 | 3.06±0.9 * | 0.9±0.19 | 0.95±0.3NS | 1.06±0.2 | 1.5±0.7 NS |
| PPARδ | 1±0.4 | 2.3±1 NS | 0.67±0.17 | 2±0.67 NS | 0.42±0.16 | 3.3±2.2 NS |
| PGC1α | 1±0.2 | 2.3±0.3 * | 1.1±0.25 | 2.5±0.8 NS | 0.87±0.4 | 1.6±0.7 NS |
| SERBP | 1±0.5 | 9.9±4.4 * | 0.57±0.2 | 1±0.5 NS | 0.39±0.25 | 2.4±1.8 NS |
| HSL | 1±0.2 | 5.3±1.9 * | 0.89±2.8 | 1.6±0.5 NS | 0.53±0.14 | 2.5±1.5 NS |
| UCP2 | 1±0.58 | 2.3±1.3NS | 0.32±0.16 | 1.56±0.56* | 0.14±0.1 | 1.5±1.4 NS |
indicates statistically significant difference between GHRKO and normal mice within analyzed tissue (P<0.05).
NS indicates lack of significance.
VISCERAL FAT REMOVAL
Blood chemistry
Analysis of fasted insulin, glucose, adiponectin and leptin in plasma indicated significant genotype/surgery interactions (P<0.02) (Table 3). Fasted insulin levels were significantly decreased after VFR in N mice only (P<0.02) (31). Interestingly, glucose levels were increased in GHRKO mice after VFR in comparison to sham-operated knockouts (P<0.01), while in N mice there was a non-significant opposite trend; i.e., reduction of glucose with VFR. For glucose levels, there was a significant genotype/VFR interaction (P<0.03) (Table 3)(31). Adiponectin level was upregulated in GHRKO mice in comparison to normal in sham-operated mice (P<0.0001). However, VFR caused a significant decrease of adiponectin level in GHRKO mice (P<0.0012) with no alteration in normal animals. Two-way ANOVA indicated significant genotype/VFR interaction for adiponectin (P<0.0078) (Table 3). Additionally, the measurement of adiponectin in adipose tissue indicated that epididymal fat has a higher level of adiponectin than subcutaneous fat in GHRKO animals (P<0.0085) (Supplement 1). Leptin levels were not different between GHRKO and normal mice in sham-operated animals, but there was a significant increase after VFR in GHRKO mice that was not seen in normal mice (Table 1). Regardless of the change in leptin level after VFR there was no alteration of food consumption in either genotype after surgery (Table 3). Plasma free fatty acids (FFA), cholesterol and triglyceride levels were not affected by VFR in either N or GHRKO animals (Table 3).
Table 3.
The levels of insulin, glucose, adiponectin, leptin, free fatty acids, triglycerides, cholesterol, food consumption and body weights in growth hormone receptor/binding protein knockout (GHRKO) and normal mice subjected to visceral fat removal or sham surgeries (n=8–10). Means ± SEM.
| N-Sham | N-VFR | GHRKO-Sham | GHRKO-VFR | |
|---|---|---|---|---|
| Body Weight (g) | 29.5±0.09a | 26.9±1.2a | 16.9±1.5a | 17.7±1.4a |
| Insulin (ng/ml) | 1.278±0.3a | 0.578±0.167b | 0.395±0.112b | 0.230±0.071b |
| Glucose (mg/dl) | 116±12a,b | 101±3.9a | 95±6.1a | 132±13b |
| Adiponectin (mg/ml) | 3693±307.6a | 3614±306.5a | 6258±344b | 4306±372a |
| Leptin (mg/ml) | 2.078±0.42a | 2.287±0.72a | 2.38±0.64a | 8.225±2.21b |
| FFA (mM) | 0.014±0.003a | 0.014±0.004a | 0.03±0.01a | 0.02±0.004a |
| Triglyceride (mg/dl) | 52.2±5.2a | 47.3±7.1a | 38.78±4.3a | 20.5±6.2a |
| Cholesterol (mg/dl) | 61.78±3.3a | 59.2±5.5a | 35.16±3.9b | 44.27±3.3b |
| Food Consumption (gram/gram of body weight) | 0.15±0.07a | 0.12±0.05a | 0.12±0.07a | 0.12±0.06a |
values that do not share the same letter in the superscript are statistically significantly different (P<0.05).
Visceral fat content
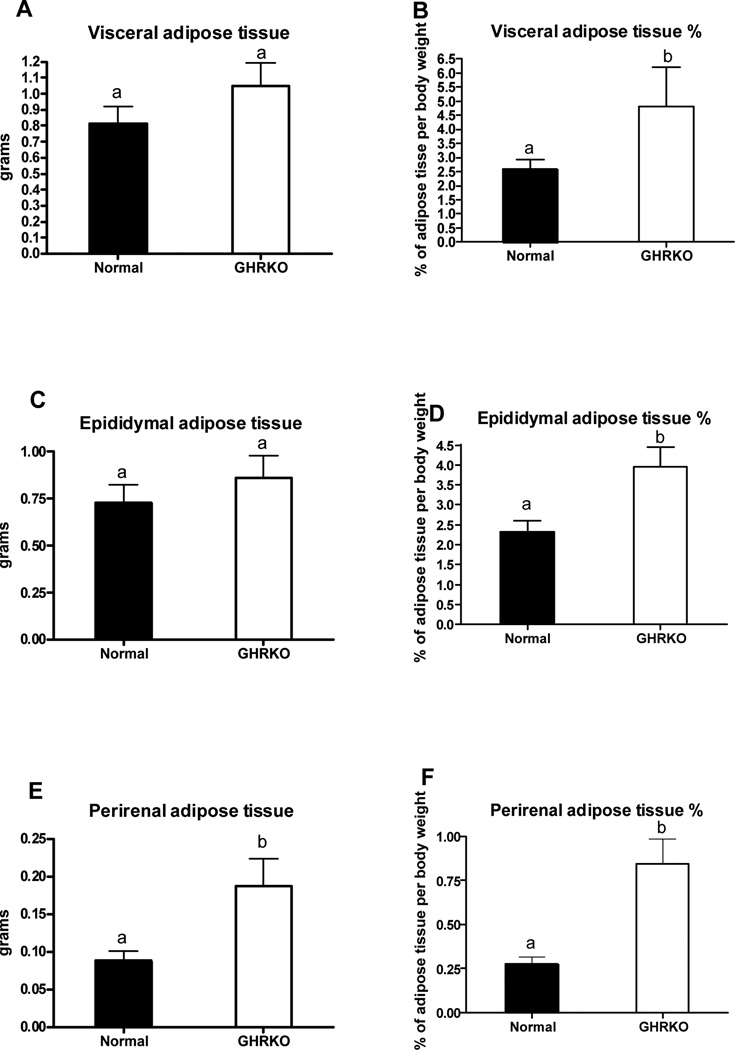
The absolute weight of visceral fat, consisting of epididymal and perinephric fat depots removed during VFR (n= 5–6 mice) did not differ between GHRKO and normal mice (Figure 1A). However, the relative amount (percent of BW) of removed visceral fat was greater in GHRKO than in normal animals (P<0.0123) (Figure 1B). The relative amount of removed epididymal fat as well as both absolute and relative amounts of removed perinephric fat were also significantly greater in GHRKO as compared to normal mice (P<0.0239; P<0.0467 and P<0.0051, respectively; Figs. 1 D–F).
Figure 1.
Visceral fat content from normal and growth hormone receptor/binding protein knockout (GHRKO) male mice presented as an absolute values and percentage of body weight (n=5–6). Means ± SEM. a,b – values that do not share the same letter in the superscript are statistically significant (P<0.05).
Insulin and glucose tolerance
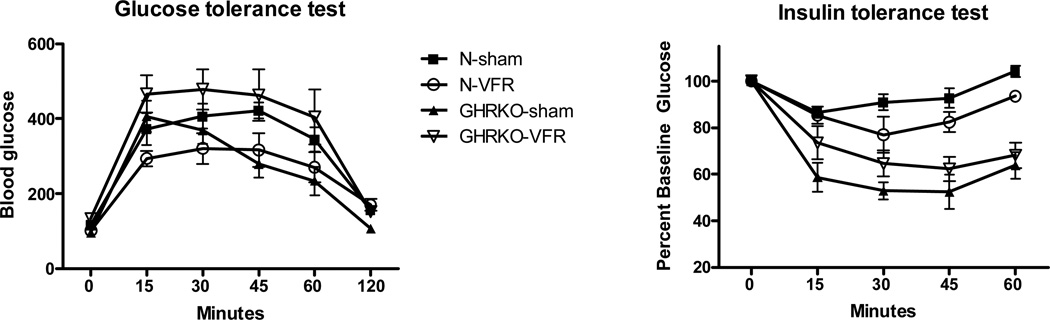
The repeated measures ANOVA test indicated significant genotype/procedure interaction for insulin tolerance test (ITT) and glucose tolerance test (GTT) (P<0.01 and P<0.018). Surgical removal of visceral fat had a tendency to improve insulin sensitivity and glucose tolerance in normal mice, but the same procedure had an opposite effect in GHRKO animals (Figure 2). These differences appeared to resolve by the end of the respective testing periods.
Figure 2.
Results of insulin tolerance test (ITT) and glucose tolerance test (GTT) in normal and growth hormone receptor/binding protein knockout (GHRKO) mice subjected to surgical visceral fat removal (VFR) or sham surgery (n=5–6).
Body temperature
A two-way ANOVA analysis of morning temperatures indicated significant genotype/intervention interaction (P<0.0154). The measurement of evening body temperature (n=5–6 mice) indicated lower temperature in GHRKO mice in comparison to normal mice (P<0.0049) with a similar trend in the morning temperatures (Supplement 1). VFR caused a decrease of both morning and evening body temperature in normal mice (P<0.0012 and P<0.0033, respectively) with no changes in GHRKO animals (Supplement 2).
The effect of VFR on insulin receptor and insulin receptor substrate in skeletal muscle
The two-way ANOVA test indicated a significant genotype/VFR interaction in IR total protein level (P<0.0140) (Supplement 3). Insulin receptor (IR) mRNA and total protein appeared to be elevated in N-VFR when compared to N-Sham mice (P<0.08 and P<0.0519, respectively) (Supplement 3 A and B). In contrast, there was a tendency for decreased mRNA and protein levels of IR in GHRKO-VFR when compared to the GHRKO-Sham group, though this decrease did not reach statistical significance. The insulin-induced activation of IR (as measured by phosphorylation at Y1158) was increased in N-VFR as compared to N-Sham animals (P<0.0047) (Supplement 3C) but not affected by VFR in GHRKO mice. The levels of IRS1 mRNA or total IRS1 protein were not altered by VFR in either genotype (Supplement 3 D and E). Phosphorylation of IRS1 at Serine307, which inhibits transmission of the insulin signal and leads to insulin resistance, was significantly decreased by VFR in N but not in GHRKO animals (P<0.0404).
Lipolysis
The in vitro analysis of lipolysis in fat pads from GHRKO and normal mice indicated that the lipolysis processes are decreased in both subcutaneous and epididymal fat pads from GHRKO mice when compared to the same fat pads from normal controls (p< 0.013 and p<0.001, respectively). There was a trend toward downregulation of lipolysis in perinephric fat from GHRKO mice when compared to normal mice, but the difference was not statistically significant (p<0.068) (Supplement 4).
Intra-hepatic and intra-muscular fat
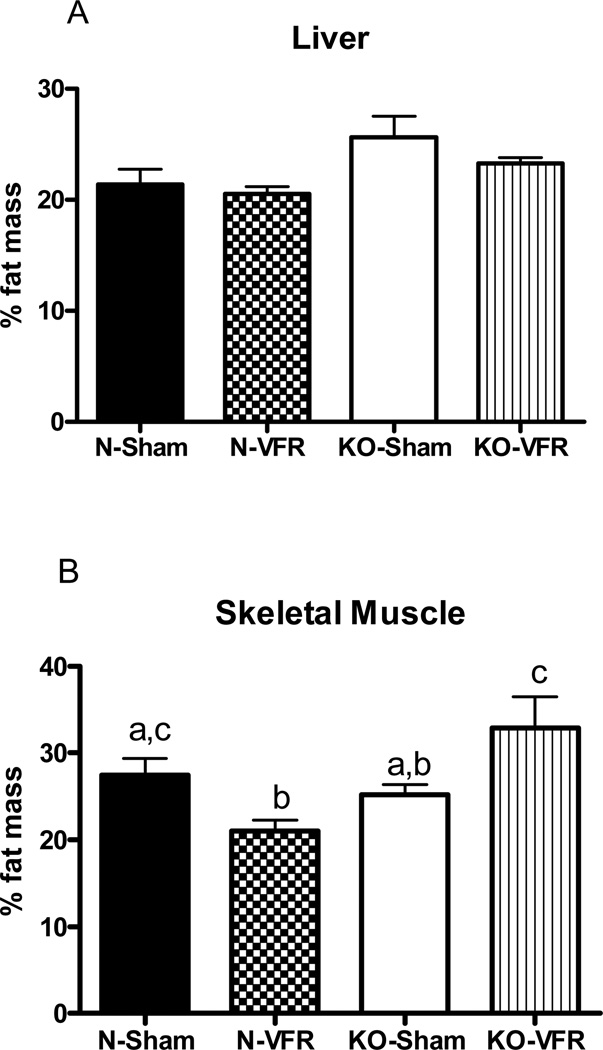
MRI analysis of tissues indicated no difference in fat accumulation in either liver or skeletal muscle when comparing sham-operated GHRKO and N mice (Figure 3). However, the two-way ANOVA indicated significant genotype/VFR interaction in skeletal muscle (P<0.002) (Figure 3B). Removing VF from N animals caused a significant decrease in fat content of skeletal muscle (P<0.01). In contrast, intramuscular fat levels were increased in GHRKO-VFR mice in comparison to GHRKO-Sham animals (P<0.047) (Figure 3B).
Figure 3.
The content of fat in (A) liver and (B) skeletal muscle from normal and growth hormone receptor/binding protein knockout (GHRKO) mice subjected to surgical visceral fat removal (VFR) or sham surgery. Results presented as a percentage of fat mass (n=8–10). Means ± SEM. a,b,c – values that do not share the same letter in the superscript are significantly different (P<0.05).
Respiratory quotient (RQ) and oxygen consumption (VO2)
Indirect calorimetry studies were conducted in the presence and in the absence of food. On the fed day, normal mice with VFR had decreased RQ compared to normal sham animals during the dark period (p=0.0197) (Figure 6) indicating a shift toward greater lipid vs. carbohydrate utilization. Conversely, GHRKO mice with VFR had increased RQ during both the dark and light period (p=0.0197 and p=0.0003 respectively) (Figure 6) compared to GHRKO sham animals. On the fasted day, normal mice with VFR had reduced RQ during the light period (p=0.0047 vs normal-sham) (Figure 6), while GHRKO mice with VFR had increased RQ during both the dark and light period (p=0.0035 and p=0.0202, respectively as compared to GHRKO-sham) (Figure 6).
Oxygen consumption, VO2 (expressed in ml O2 per unit BW) was not altered after VFR in either N or GHRKO mice, except for a significant increase of VO2 in the GHRKO-VFR group for a period of three hours on the fed day (P<0.04) (Supplement 6).
DISCUSSION
The key conclusion emerging from the present study is that in the absence of GH signals, function of visceral adipose tissue and its metabolic impact are profoundly altered. Our previous findings (6, 9–14) imply that there may be different roles of the adipose tissue as an endocrine organ in GHRKO mice in comparison to their normal controls. The differences could involve an altered pattern of adipokine production and release, and indeed plasma adiponectin levels are elevated in GHRKO mice. In support of this interpretation, the levels of adiponectin in epididymal fat from GHRKO mice were higher than the levels measured in subcutaneous fat from the same animals or in the subcutaneous or epididymal fat from normal controls. Subcutaneous fat is believed to be an important source of adiponectin present in the circulation. However, high levels of adiponectin in epididymal fat of GHRKO mice would support the hypothesis that epididymal fat from GHRKO mice has different biological function than the same adipose tissue in normal controls. Functional differences between visceral fat of normal and GHRKO mice also include reduced levels of resistin and IL-6 in perinephric fat of GHRKOs and downregulation of IL-6 in the epididymal fat of these animals. Since adiponectin is anti-inflammatory and promotes insulin sensitivity while IL-6 is proinflammatory, the observed alterations in adipokines levels in the visceral fat of GHRKO vs. normal mice support the hypothesis that these differences represent an important mechanism that regulates whole-body insulin sensitivity and health of these long-living animals.
Additionally the analysis of genes involved in lipid metabolism indicated that relative expression of IR, PPARγ, PPARα, PGC1α, SERBP and HSL was increased in epididymal fat from GHRKO as compared to normal mice without significant genotype effects in other fat depots. This indicates increased lipogenesis in GHRKO mice, and could explain increased fat accumulation in these animals. However, GHRKO mice have also increased fat accumulation at subcutaneous and perinephric sites where we did not find any alterations in the expression levels of the same genes. Presumably, differences in the metabolism and secretory function of epididymal fat are among important mechanisms of differential regulation of insulin sensitivity in GHRKO vs. normal mice with perinephric fat having no or a lesser role. Based on the present data and previous observation that GHRKO mice do not benefit from calorie restriction (6), we hypothesize that elevation of anti-inflamatory adiponectin and expression of genes promoting insulin sensitivity together with suppression of IL-6 levels represent mechanisms linking alterations in intra-abdominal adipose tissue biology with whole-body insulin sensitivity in these long-living animals. Following these findings we decided to compare the effects of visceral fat removal in GHRKO and normal animals.
The present results demonstrate that surgical removal of visceral fat produces disparate, in many cases opposite effects in the genetically GH-resistant GHRKO as compared to normal mice. The net result is improvement of insulin sensitivity and induction of metabolic traits associated with increased longevity in normal animals and induction of insulin resistance and detrimental metabolic changes in GHRKO animals. These largely unexpected findings indicate that secretory activity of visceral fat of GHRKO mice contributes to the metabolic profile that favors extended longevity of these mutants. They also provide a likely explanation for the coexistence of obesity with enhanced insulin sensitivity and increased lifespan in these animals. Moreover, the present findings identify a likely reason why GHRKO mice do not benefit from calorie restriction by improved insulin signaling or a further extension of longevity.
In addition to their remarkable longevity (5,6), GHRKO mice have extended “healthspan” as indicated by protection from age-related decline of cognitive function and by reduced incidence and delayed occurrence of cancer (32). Similar findings have been recorded in hypopituitary Ames and Snell dwarf mice which are GH deficient (33). The physiological alterations observed in GH deficiency and GH resistance resemble in many ways the effects of calorie restriction (CR). However, the lifespan of Ames dwarf mice can be further extended by CR (1), suggesting that CR and GH deficiency do not affect longevity through identical mechanisms. Surprisingly, the GH-resistant GHRKO animals gained no further life extension benefit from CR (6). This unexpected finding raised a possibility of substantial overlap between mechanism responsible for extension of longevity by CR and by GH resistance. However, in contrast to animals subjected to CR, GHRKO mice are obese in comparison to normal littermates (14;15) and yet are insulin sensitive and long-lived. Berryman et al. determined that the obesity of GHRKO mice was primarily due to increased subcutaneous fat depots with a trend toward increased visceral obesity in GHRKO mice at the age of 2 years (15). In our colony with a heterogeneous genetic background (34), GHRKO mice have similarly increased subcutaneous fat but also exhibit increased visceral obesity that is usually associated with increased insulin resistance and risk of diabetes. This sharply contrasts with insulin sensitivity and extension of healthspan and lifespan in GHRKO animals (6;33;34;35). These counterintuitive findings and differences in biology of adipose tissue in GHRKO and normal mice lead us to speculate that the role of visceral fat (VF) in the regulation of insulin/glucose metabolism in the GHRKO mice differs from its role in normal animals.
Decreased body temperature after VFR in normal animals resembles the effect observed in CR mice, which would agree with the hypothesis that VFR mimics CR (26;29;35). Supporting this hypothesis, the lack of the body temperature alteration in GHRKO mice after VFR coincides with the previous findings that responses to CR are greatly attenuated in GHRKO mice (6;30). More importantly, the disparate effects of VFR on ITT, GTT, insulin and glucose levels(31) in GHRKO as compared to normal mice indicate that eliminating or decreasing the amount of VF tissue by surgical or dietary interventions is not beneficial to GHRKO mice.
Consistent with the lipolytic actions of GH, lipolysis measured in vitro in adipose tissue from GHRKO mice was reduced in comparison to the values measured in fat derived from normal animals. Importantly, there was no difference in lipolysis levels between subcutaneous and epididymal fat from GHRKO mice, while epididymal fat from normal mice had much greater lipolysis level than subcutaneous fat from the same animals. It could be concluded that in terms of lipolysis, epididymal fat from GHRKO mice shares some characteristics with subcutaneous fat. Additional studies are needed to identify mechanisms responsible for these somewhat counterintuitive findings.
There is a well established association between metabolic disorders and fat accumulation at ectopic sites. Surprisingly, no differences were detected in intra-muscular or intra-hepatic fat accumulation between normal and highly insulin-sensitive GHRKO mice. However, surgical VFR promoted reduction of intramuscular fat in normal mice, which likely contributed to the observed improvement in whole-body insulin sensitivity and glucose tolerance. Unexpectedly, the same procedure in GHRKO animals, promoted an increased accumulation of lipids in skeletal muscle, which may be one of the key causes of decreased whole-body insulin sensitivity in GHRKO-VFR mice. Apparently VF influences fat distribution between the adipose tissue and tissues that normally store small amounts of lipid.
The analysis of the insulin receptor (IR) activation in skeletal muscle indicated that in normal animals VFR enhances responses to insulin in this tissue. Decrease of the inhibitory IRS-1 phosphorylation at IRS1307ser, after VFR in normal mice also implies improved insulin signaling and resembles findings in CR mice (6). Importantly, muscle levels of p-IRS1307ser in GHRKO mice were not affected by VFR (the present study) or by CR (6, 30). The lack of alterations in the insulin signaling pathway in skeletal muscle of GHRKO mice after VFR corresponds to the lack of improvement in whole-body insulin sensitivity.
Following the demonstration of different responses of glucose metabolism responses to VFR in normal and GHRKO mice, we investigated the impact of this intervention on metabolic rate and transitions of fuel selection between fat and carbohydrate oxidations by measuring VO2 and RQ. RQ is a dimensionless ratio comparing the volume of carbon dioxide (VCO2) an organism produces over a given time, to its oxygen consumption (VO2). This ratio (RQ = VCO2 /VO2) gives an estimate of the primary metabolic fuel source, which is typically either fat (RQ = 0.7) or carbohydrate (RQ = 1) oxidation. Thus, RQ varies inversely with lipid oxidation; a higher fasting RQ, which indicates lowered fat oxidation, is linked to body weight gain, metabolic inflexibility and insulin resistance (36). In the normal mouse, VFR decreased RQ, indicating increased fat oxidation. This novel effect of VFR suggests that the visceral fat that was removed was somehow impeding fat metabolism in the normal mice. Interestingly, the opposite was seen in the GHRKO mouse after VFR; the RQ of GHRKO-VFR mice was increased, indicating increased carbohydrate utilization compared to GHRKO sham animals. This suggests that visceral fat in GHRKO mice promotes the metabolism of fats, perhaps through adiponectin production.
In summary, surgical removal of visceral fat—generally considered to be “bad fat”—in normal animals broadly mimics the effects of CR in agreement with previous studies (35). Calorie restriction acts on adipose tissue to decrease fat content, but its actions are much more complex at the whole-animal level. The present study indicates that surgical VFR also produces complex alterations in whole-body insulin sensitivity/glucose regulation, oxygen consumption, respiratory quotient, ectopic fat distribution and insulin signaling in skeletal muscle. Absence of beneficial effects of VFR in GHRKO mice indicates that the same endocrine organ plays a different role in GHRKO and N mice. Indeed the present findings strongly suggest that VF has an unexpectedly important and positive role in regulating insulin action and perhaps also longevity in GHRKO mice. Finally, disparate effects of VFR in normal and GHRKO mice could explain why CR, known to target mainly adipose tissue, affects insulin sensitivity and longevity in normal but not in GHRKO mice.
Collectively, the present findings suggest that GH signals in adipose tissue could be among key regulators of insulin action and longevity, and targeting suppression of this specific pathway in only white adipose tissue might produce beneficial CR-like effects in mammals. These findings, together with previous findings on alterations in expression of various genes in different organs of long-living mutants and CR animals (9;11–13;37–43), indicate that a search for pharmaceutical interventions specifically targeting VF rather than working globally could be rewarding in terms of metabolic, anti-diabetic and longevity benefits. It might also reduce the wide list of potentially undesirable side effects.
MATERIALS AND METHODS
Experimental animals and animal maintenance
Normal and GHRKO male mice used in this study were produced in our breeding colony and developed by crossing 129Ola/BALB/c normal (GHR+/−) animals generously provided by Dr. J.J. Kopchick with mice derived from crosses of C57BL/6J and C3H/J strains; these mice were then maintained as a closed colony with inbreeding minimized by avoiding brother × sister mating (34). All animal protocols for this study were approved by the Southern Illinois University Laboratory Animal Care and Use Committee. The animals were housed under temperature- and light-controlled conditions (20–23°C, 12-hr light/12-hr dark cycle) and were provided ad libitum with nutritionally balanced diet (Rodent Laboratory Chow 5001; 23.4% protein, 4.5% fat, 5.8% crude fiber; LabDiet PMI Feeds, Inc., St. Louis, MO). To produce GHRKO (−/−) mice, knockout (−/−) males were mated with heterozygous (+/−) females. Heterozygous normal (+/−) males were used as controls based on previous data indicating that there are no significant phenotypic differences between normal homozygous and heterozygous mice (4).
Surgical visceral fat removal
At the age of about 5 months, GHRKO and normal littermates (N) were subjected to visceral fat removal (VFR) or sham (S) surgery. All animals in these experiments were males. The animals were anesthetized with ketamine/xylazine, shaved and prepared in the usual sterile fashion. Additionally, animals were supplied with ibuprofen in drinking water starting 2 days before and up to 3 days after the surgery. In the VFR group, for technical reasons only the major fat pads epididymal and perinephric were removed, with no attempt to remove mesenteric or omental fat. The epididymal fat pads were removed using blunt dissection through a vertical midline incision, and then perinephric fat pads were removed via flank incisions. We removed as much epididymal or pherinephric fat as was possible without compromising blood supply to the testes and to the adrenals. For sham operations, the abdominal cavity and both sides of the back were incised and the VF was mobilized, but not removed. We performed three independent experiments at different time points with VFR to be able to measure different parameters.
Animal testing
Ten days post-surgery, animals were subjected to insulin tolerance tests (ITT) as described below (n=5–6). Fifteen days after surgery, glucose tolerance tests (GTT) were performed as described below (n=5–6).
Tissue collection
At 55 days after VFR, after fasting over-night (~14 h) animals were anesthetized with ketamine/xylazine. Approximately 200–300 µl of blood was collected by cardiac puncture. After bleeding, half of the animals from each experimental group were injected with a high dose of insulin (10IU/KG of body weight) or saline through the liver portal vein to stimulate the insulin signaling pathway following previously described protocol (n=8–10) (30). Exactly 2 minutes after insulin injection and sacrifice muscle tissues were collected and kept on dry ice until moved to −80° C freezer. At the time of tissue collection 55 days after surgery of visceral fat removal, there was no visible fat regrowth.
Assessment of blood chemistry
Plasma was obtained from blood collected by cardiac puncture and was used for assessment of adiponectin using Mouse Adiponectin ELISA and leptin using Mouse Leptin ELISA following manufacturer’s protocols (Linco Research Inc., St. Charles, MO and IDS, Inc., Fountain Hills, AZ).
Insulin tolerance test (ITT) and glucose tolerance test (GTT)
For ITT, non-fasted mice were injected i.p. with 0.75 IU insulin per kilogram of body weight. Blood glucose levels were measured at 0, 15, 30, and 60 minutes using a OneTouch Ultra glucometer. The data for ITT are presented as a percentage baseline of glucose. Fourteen hours fasted mice underwent GTT by intraperitoneal (i.p.) injection with 2 g of glucose per kilogram of body weight. Blood glucose levels were measured at 0, 15, 30, 45, 60, and 120 minutes.
Body temperature measurements
Body temperature was measured using Implantable Programmable Temperature Transponders (IPTT) provided by Bio Medic Data Systems (BMDS) Inc., Seaford, DE. IPTT 300 transponders were implanted subcutaneously in the inter-scapular region using transponder trocor kit. Temperatures from the transponders were read using the DAS-6007 Smart Probe.
BMDS radio-telemetric probe reader
After transponder implantation, mice were allowed to heal for two weeks before measurements were taken. Mice were acclimated to the probes by taking mock measurements not used for data. Measurements were taken at two different time-points: 6AM and 6PM. Data were compiled and expressed as averages of each time-point.
Real-time PCR
mRNA expression was analyzed by real-time PCR (RT-PCR) using the Smart Cycler instrument (Cepheid, Sunnyvale, CA) with iQ™ SYBR Green Supermix (BIO-RAD). Details of the procedure and the list of primers were reported previously (11;12;38;40;41).
Lipolysis analysis
Subcutaneous, epididymal and perirenal adipose tissues were surgically removed from 9–9.5 month old male GHRKO and normal mice. The tissues were rinsed three times with PBS buffer. Tissue pieces (about 100 mg) were incubated in Dulbecco’s modified Eagle medium (GIBCO, invitrogen Corp., Carlsbad, CA) with or without 10% FBS (GIBCO, invitrogen Corp., Carlsbad, CA) at 37°C. Aliquots of the medium were collected after being cultured for 24 hours and investigated for free glycerol content using commercial Kit (Sigma, St Louis, MO)
Enzyme-linked immunosorbant assay for insulin receptor (IR) and insulin receptor substrate 1 (IRS-1) in skeletal muscle
The level of total IR and IRS1, and the phosphorylated forms (pY1158 IR and IRS1307ser) were analyzed by commercially available ELISA kit (Invitrogen, Carlsbad, CA) according to provided protocol.
Magnetic resonance imaging (MRI)
Frozen tissue samples were thawed to 4° C and analyzed using an EchoMRI 3-in-1 composition analyzer (Echo Medical Systems, Houston TX). Tissue composition was determined by the formula [fat mass/(fat mass + lean mass)].
Indirect calorimetry
Adult (6–7 month-old) male GHRKO and normal littermates (N) that had been subjected to either visceral fat removal (VFR) or sham (S) surgery (n=8 per phenotype) were measured by indirect calorimetry using the PhysioScan Metabolic System (AccuScan Instruments, Inc., Columbus, OH). This system utilizes zirconia and infrared sensors to monitor oxygen (O2) and carbon dioxide (CO2), respectively, inside of respiratory chambers in which individual mice were tested. All comparisons are based on animals studied simultaneously in eight different chambers connected to the same O2 and CO2 sensors in an effort to minimize the effect of environmental variations and calibration on data. After a 24-hour acclimation period, mice were monitored in the metabolic chambers for 24 hours with ad libitum access to standard chow (Laboratory Diet 5001) and water; and then for a second 24-hour period without food. Gas samples were collected and analyzed every five minutes per animal, and the data were averaged for each hour. Output parameters include oxygen consumption (VO2, mL/kg/min) and respiratory quotient (RQ, VCO2/ VO2).
Statistical analysis
A simple one-way ANOVA was used to analyze visceral fat deposition. Body temperature, blood chemistry, intra-hepatic and intra-muscular fat deposition, and insulin receptor mRNA and protein data were analyzed using two-way ANOVAs. A three-way ANOVA was used to analyze phosphorylation levels of IR and IRS1. A repeated measures ANOVA was used to determine the interaction and main effects for ITT and GTT data followed by Fisher’s LSD tests for pairwise comparisons. Student t-test was used to analyze RQ and two-way repeated measures ANOVA was used to analyze VO2. α was set at 0.05 for determination of significance, and all values are reported as mean ± SEM throughout the figures and text.
Supplementary Material
Supplement 1
The levels of adiponectin in subcutaneous and epididymal fat tissues from growth hormone receptor/binding protein knockout (GHRKO) and normal mice (n=8–10). Means ± SEM. a,b, – values that do not share the same letter in the superscript are significantly different (P<0.05).
Supplement 2
Body temperatures in normal and growth hormone receptor/binding protein knockout (GHRKO) mice subjected to surgical visceral fat removal (VFR) or sham surgery (n=5–6). Means ± SEM. a,b, – values that do not share the same letter in the superscript are significantly different (P<0.05).
Supplement 3
The level of proteins and mRNA related to insulin signaling in skeletal muscle tissue of normal and growth hormone receptor/binding protein knockout (GHRKO) mice subjected to surgical visceral fat removal (VFR) or sham surgery (n=8–10). Means ± SEM. a,b,c – values that do not share the same letter in the superscript are significantly different (P<0.05). (A) insulin receptor (IR)-mRNA (B) IR-total protein (C) IR- phosphorylated at site tyrosine 1158 (IR-pY1158), (D) Insulin receptor substrate 1 (IRS1)- mRNA, (E) IRS1- total protein (F) IRS1 phosphorylated at site serine 307 (IRS1-pS307)
Supplement 4
The levels of in vitro lipolysis in subcutaneous (Sc), epididymal (Ep) and perinephric (Ph) fat tissues from growth hormone receptor/binding protein knockout (GHRKO) and normal mice. Means ± SEM. a,b, – values that do not share the same letter in the superscript are significantly different (P<0.05).
Supplement 5
The levels of respiratory quotient (RQ) in fasted (A,B) or fed (C,D) GHRKO and normal mice after visceral fat removal (VFR) or sham surgery (n=8–10).
Supplement 6
Oxygen consumption (VO2) in GHRKO and normal mice during fasted and fed time (n=8–10).
Acknowledgments
Supported by NIA, AG032290, AG 19899, AG031736 and U19 AG023122, The Ellison Medical Foundation, Central Research Committee of Southern Illinois University School of Medicine, EAM’s SIU and Polish Ministry of Science and Higher Education (N N401 042638). We would also like to thank Dr. Nir Barzilai for providing the surgery protocol and Steve Sandstrom for editorial assistance.
Reference List
- 1.Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001 Nov 22;414(6862):412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- 2.Bartke A. Insulin and aging. Cell Cycle. 2008 Nov 1;7(21):3338–3343. doi: 10.4161/cc.7.21.7012. [DOI] [PubMed] [Google Scholar]
- 3.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996 Nov 7;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 4.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003 Sep;144(9):3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 5.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003 Feb 28;299(5611):1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 6.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006 May 16;103(20):7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000 Jul;141(7):2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 8.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997 Oct 2;337(14):986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology. 2005 Feb;146(2):851–860. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- 10.Bartke A, Masternak MM, Al-Regaiey KA, Bonkowski MS. Effects of dietary restriction on the expression of insulin-signaling-related genes in long-lived mutant mice. Interdiscip Top Gerontol. 2007;35:69–82. doi: 10.1159/000096556. [DOI] [PubMed] [Google Scholar]
- 11.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Caloric restriction results in decreased expression of peroxisome proliferator-activated receptor superfamily in muscle of normal and long-lived growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2005 Oct;60(10):1238–1245. doi: 10.1093/gerona/60.10.1238. [DOI] [PubMed] [Google Scholar]
- 12.Masternak MM, Al-Regaiey KA, Bonkowski MS, Panici JA, Bartke A. Effect of every other day feeding diet on gene expression in normal and in long-lived Ames dwarf mice. Exp Gerontol. 2005 Jun;40(6):491–497. doi: 10.1016/j.exger.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Masternak MM, Bartke A. PPARs in Calorie Restricted and Genetically Long-Lived Mice. PPAR Res. 2007;2007:28436. doi: 10.1155/2007/28436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonkowski MS, Pamenter RW, Rocha JS, Masternak MM, Panici JA, Bartke A. Long-lived growth hormone receptor knockout mice show a delay in age-related changes of body composition and bone characteristics. J Gerontol A Biol Sci Med Sci. 2006 Jun;61(6):562–567. doi: 10.1093/gerona/61.6.562. [DOI] [PubMed] [Google Scholar]
- 15.Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004 Aug;14(4):309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Carey VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses' Health Study. Am J Epidemiol. 1997 Apr 1;145(7):614–619. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 17.Nicklas BJ, Cesari M, Penninx BW, et al. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc. 2006 Mar;54(3):413–420. doi: 10.1111/j.1532-5415.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 18.Ross R, Berentzen T, Bradshaw AJ, et al. Does the relationship between waist circumference, morbidity and mortality depend on measurement protocol for waist circumference? Obes Rev. 2008 Jul;9(4):312–325. doi: 10.1111/j.1467-789X.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005 Mar;81(3):555–563. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 20.Misra A, Garg A, Abate N, Peshock RM, Stray-Gundersen J, Grundy SM. Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obes Res. 1997 Mar;5(2):93–99. doi: 10.1002/j.1550-8528.1997.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 21.Snijder MB, Dekker JM, Visser M, et al. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr. 2003 May;77(5):1192–1197. doi: 10.1093/ajcn/77.5.1192. [DOI] [PubMed] [Google Scholar]
- 22.Tanko LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003 Apr 1;107(12):1626–1631. doi: 10.1161/01.CIR.0000057974.74060.68. [DOI] [PubMed] [Google Scholar]
- 23.Thorne A, Lonnqvist F, Apelman J, Hellers G, Arner P. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord. 2002 Feb;26(2):193–199. doi: 10.1038/sj.ijo.0801871. [DOI] [PubMed] [Google Scholar]
- 24.Klein S, Fontana L, Young VL, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004 Jun 17;350(25):2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 25.Langendonk JG, Kok P, Frolich M, Pijl H, Meinders AE. Decrease in visceral fat following diet-induced weight loss in upper body compared to lower body obese premenopausal women. Eur J Intern Med. 2006 Nov;17(7):465–469. doi: 10.1016/j.ejim.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Barzilai N, Banerjee S, Hawkins M, Chang CJ, Chen W, Rossetti L. The effect of age-dependent increase in fat mass on peripheral insulin action is saturable. J Gerontol A Biol Sci Med Sci. 1998 Mar;53(2):B141–B146. doi: 10.1093/gerona/53a.2.b141. [DOI] [PubMed] [Google Scholar]
- 27.Dubuc PU. The development of obesity, hyperinsulinemia, and hyperglycemia in ob/ob mice. Metabolism. 1976 Dec;25(12):1567–1574. doi: 10.1016/0026-0495(76)90109-8. [DOI] [PubMed] [Google Scholar]
- 28.Rebuffe-Scrive M, Surwit R, Feinglos M, Kuhn C, Rodin J. Regional fat distribution and metabolism in a new mouse model (C57BL/6J) of non-insulin-dependent diabetes mellitus. Metabolism. 1993 Nov;42(11):1405–1409. doi: 10.1016/0026-0495(93)90190-y. [DOI] [PubMed] [Google Scholar]
- 29.Gabriely I, Ma XH, Yang XM, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002 Oct;51(10):2951–2958. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- 30.Bonkowski MS, Dominici FP, Arum O, et al. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One. 2009;4(2):e4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Gesing A, Masternak MM, Wang F, Karbownik-Lewinska M, Bartke A. Deletion of growth hormone receptor gene but not visceral fat removal decreases expression of apoptosis-related genes in the kidney-potential mechanism of lifespan extension. Age (Dordr) 2011 Mar 23; doi: 10.1007/s11357-011-9232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeno Y, Hubbard GB, Lee S, et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009 May;64(5):522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003 Apr;58(4):291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 34.Panici JA, Wang F, Bonkowski MS, et al. Is Altered Expression of Hepatic Insulin-Related Genes in Growth Hormone Receptor Knockout Mice Due to GH Resistance or a Difference in Biological Life Spans? J Gerontol A Biol Sci Med Sci. 2009 Aug 25; doi: 10.1093/gerona/glp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muzumdar R, Allison DB, Huffman DM, et al. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008 Jun;7(3):438–440. doi: 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snitker S, Tataranni PA, Ravussin E. Respiratory quotient is inversely associated with muscle sympathetic nerve activity. J Clin Endocrinol Metab. 1998 Nov;83(11):3977–3979. doi: 10.1210/jcem.83.11.5265. [DOI] [PubMed] [Google Scholar]
- 37.Al-Regaiey KA, Masternak MM, Bonkowski MS, Panici JA, Kopchick JJ, Bartke A. Effects of caloric restriction and growth hormone resistance on insulin-related intermediates in the skeletal muscle. J Gerontol A Biol Sci Med Sci. 2007 Jan;62(1):18–26. doi: 10.1093/gerona/62.1.18. [DOI] [PubMed] [Google Scholar]
- 38.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Effects of caloric restriction and growth hormone resistance on the expression level of peroxisome proliferator-activated receptors superfamily in liver of normal and long-lived growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2005 Nov;60(11):1394–1398. doi: 10.1093/gerona/60.11.1394. [DOI] [PubMed] [Google Scholar]
- 39.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Effects of caloric restriction on insulin pathway gene expression in the skeletal muscle and liver of normal and long-lived GHR-KO mice. Exp Gerontol. 2005 Aug;40(8–9):679–684. doi: 10.1016/j.exger.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Caloric restriction and growth hormone receptor knockout: effects on expression of genes involved in insulin action in the heart. Exp Gerontol. 2006 Apr;41(4):417–429. doi: 10.1016/j.exger.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci. 2009 May;64(5):516–521. doi: 10.1093/gerona/glp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Al-Regaiey KA, Masternak MM, Bartke A. Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. J Gerontol A Biol Sci Med Sci. 2006 Apr;61(4):323–331. doi: 10.1093/gerona/61.4.323. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Masternak MM, Al-Regaiey KA, Bartke A. Adipocytokines and the regulation of lipid metabolism in growth hormone transgenic and calorie-restricted mice. Endocrinology. 2007 Jun;148(6):2845–2853. doi: 10.1210/en.2006-1313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1
The levels of adiponectin in subcutaneous and epididymal fat tissues from growth hormone receptor/binding protein knockout (GHRKO) and normal mice (n=8–10). Means ± SEM. a,b, – values that do not share the same letter in the superscript are significantly different (P<0.05).
Supplement 2
Body temperatures in normal and growth hormone receptor/binding protein knockout (GHRKO) mice subjected to surgical visceral fat removal (VFR) or sham surgery (n=5–6). Means ± SEM. a,b, – values that do not share the same letter in the superscript are significantly different (P<0.05).
Supplement 3
The level of proteins and mRNA related to insulin signaling in skeletal muscle tissue of normal and growth hormone receptor/binding protein knockout (GHRKO) mice subjected to surgical visceral fat removal (VFR) or sham surgery (n=8–10). Means ± SEM. a,b,c – values that do not share the same letter in the superscript are significantly different (P<0.05). (A) insulin receptor (IR)-mRNA (B) IR-total protein (C) IR- phosphorylated at site tyrosine 1158 (IR-pY1158), (D) Insulin receptor substrate 1 (IRS1)- mRNA, (E) IRS1- total protein (F) IRS1 phosphorylated at site serine 307 (IRS1-pS307)
Supplement 4
The levels of in vitro lipolysis in subcutaneous (Sc), epididymal (Ep) and perinephric (Ph) fat tissues from growth hormone receptor/binding protein knockout (GHRKO) and normal mice. Means ± SEM. a,b, – values that do not share the same letter in the superscript are significantly different (P<0.05).
Supplement 5
The levels of respiratory quotient (RQ) in fasted (A,B) or fed (C,D) GHRKO and normal mice after visceral fat removal (VFR) or sham surgery (n=8–10).
Supplement 6
Oxygen consumption (VO2) in GHRKO and normal mice during fasted and fed time (n=8–10).