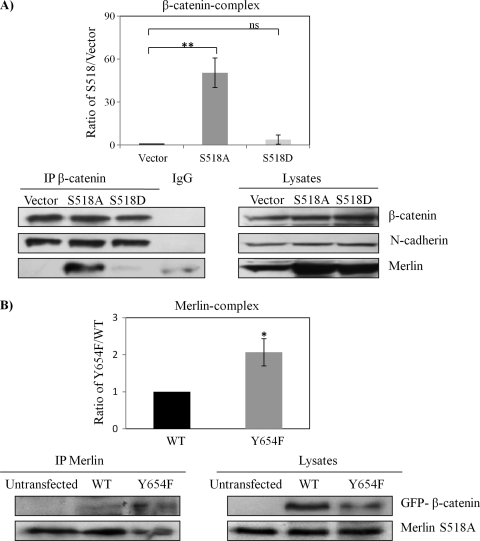
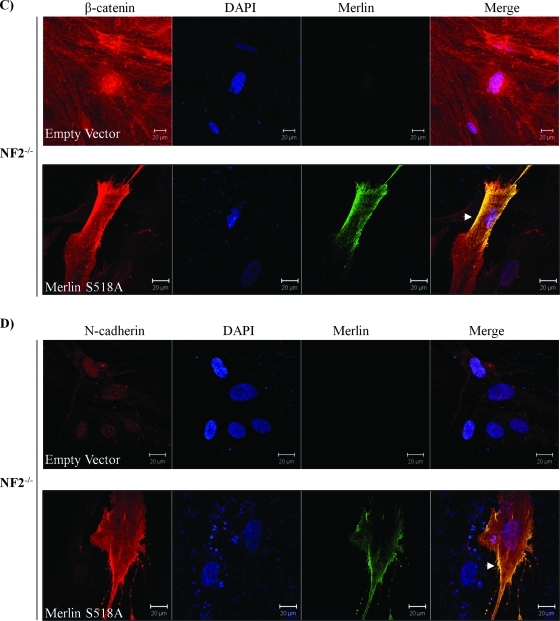
Figure 5.
Only Merlin S518A forms a complex with β-catenin and relocates AJs complex to the plasma membrane. (A) β-Catenin forms a complex with Merlin-S518A not Merlin S518D. HEK293T cells are transfected with empty vector, Merlin-S518A, and Merlin-S518D. Endogenous β-catenin complex was immunoprecipitated (IP) after treatment with pervanadate to reserve the phosphorylation status of β-catenin. IgG mouse without anti-β-catenin antibody served as a control. Immunoprecipitates were blotted for β-catenin. Merlin, N-cadherin, and RhoGDI served as controls. Densities of bands were quantified and compared. The protein level of merlin in the β-catenin complex was compared between Merlin S518 mutants and empty vector after being corrected with their count parts in total lysates. Error bars represent the mean ± SEM. n = 3. **P < .01. ns indicates no significant difference. (B) Merlin-S518A preferentially interacts with β-catenin-Y654F. Merlin-S518A stable line (HEK293T cells) was transfected with GFP-β-catenin WT and GFP-β-catenin-Y654F (Y654F), and untransfected cells. Merlin complex was immunoprecipitated and blotted for β-catenin and merlin. Densities of bands were quantified and compared. The protein level of β-catenin in the merlin complex was compared between WT and Y654F after being corrected with their count parts in total lysates. Error bars represent the mean ± SEM. n = 3. *P < .05. (C, D) Merlin-S518A relocates β-catenin and N-cadherin to the plasma membrane in schwannoma cells. Cells were transfected with either empty vector or Merlin-S518A. Immunostaining was carried out for merlin and β-catenin (C) or merlin and N-cadherin (D). DAPI was used as nuclear maker. The potential AJ sites are indicated by arrows. Scale bar, 20 µm.