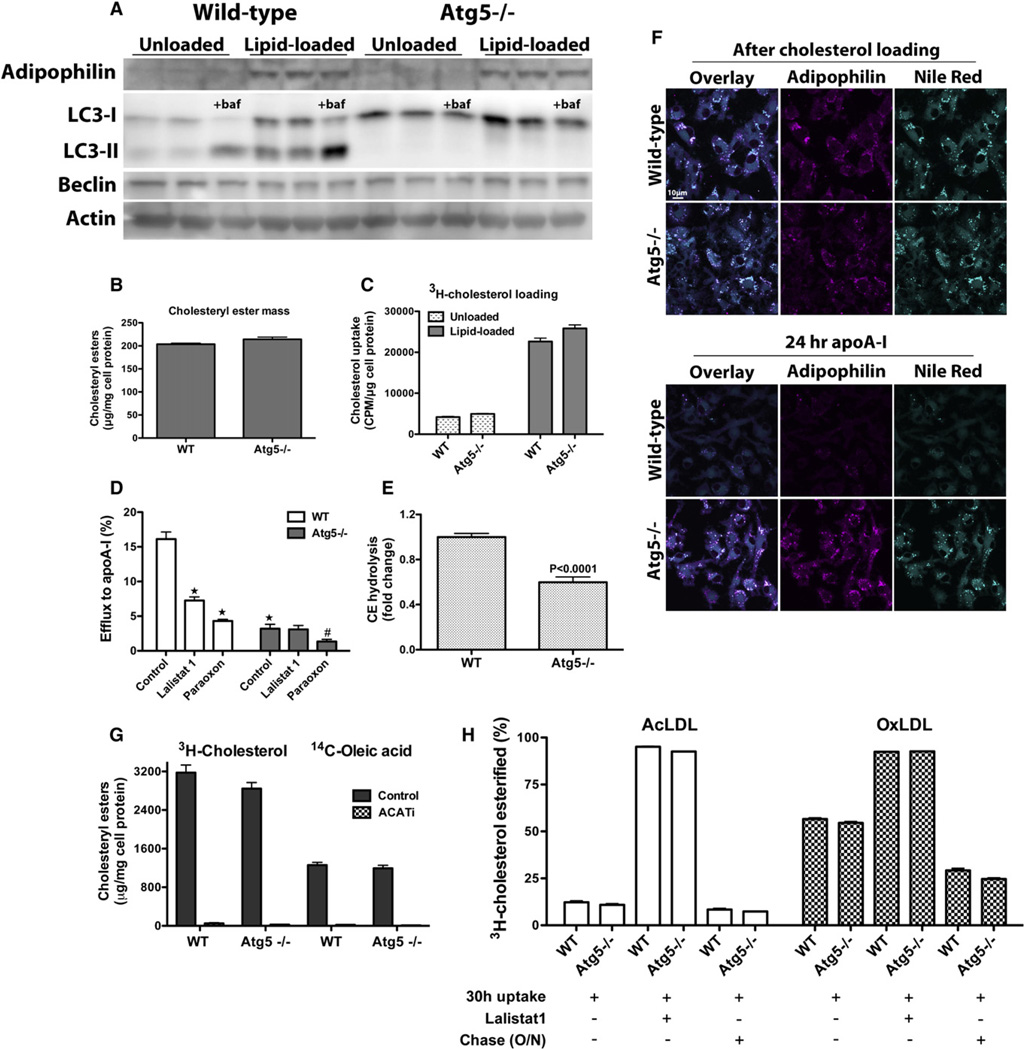
Figure 4. Ablation of Autophagy Impairs LD Delivery to Lysosomes for LAL-Mediated CE Hydrolysis in Macrophage Foam Cells.
(A) Atg5 deletion in macrophages impairs LC3-I maturation to LC3-II, and autophagy cannot ensue.
(B and C) Cholesterol loading is similar in WT and Atg5−/− macrophages, as recorded by mass measurements (B) and 3H-cholesterol uptake (C).
(D) Cholesterol efflux to apoA-I is impaired in Atg5−/− lipid-loaded macrophages; LAL inhibition reduces cholesterol efflux in WT, but not Atg5−/−, macrophages. ★p < 0.0001 relative to WT control, and #p < 0.05 relative to Atg5−/− control.
(E) CE hydrolysis in lipid-loaded WT and Atg5−/− macrophages over 24 hr in the presence of apoA-I, expressed as a fold change relative to the WT control (cellular CE mass was measured before and after cholesterol efflux, and resulting percent hydrolysis was normalized to that of WT).
(F) Autophagy-deficient macrophages exhibit impaired LD catabolism. Lipid-loaded WT or Atg5−/− macrophages were immediately fixed for fluorescence microscopy after AcLDL loading or after a 24 hr incubation with media containing lipid-poor apoA-I in the presence of an ACAT inhibitor. Neutral lipids were stained with Nile Red.
(G) AcLDL-derived 3H-cholesterol is esterified to 14C-oleic acid to the same extent in WT and Atg5−/− macrophages. In the absence of the ACATi, esterification of the 3H and 14C labels parallels each other and is equivalent in WT and Atg5−/− cells. When the ER-resident ACAT is inhibited by ACATi, esterification of both the 3H and 14C labels is abolished.
(H) Degradation of lipoprotein 3H-cholesteryl oleate occurs at the same rate in WT and Atg5−/− macrophages. Macrophages were loaded with AcLDL or OxLDL containing 3H-cholesteryl oleate in the presence or absence of Lalistat 1 to inhibit LAL, followed by an O/N equilibration or not. All experiments were performed in the presence of the ACATi to prevent re-esterification of the liberated 3H-cholesterol.