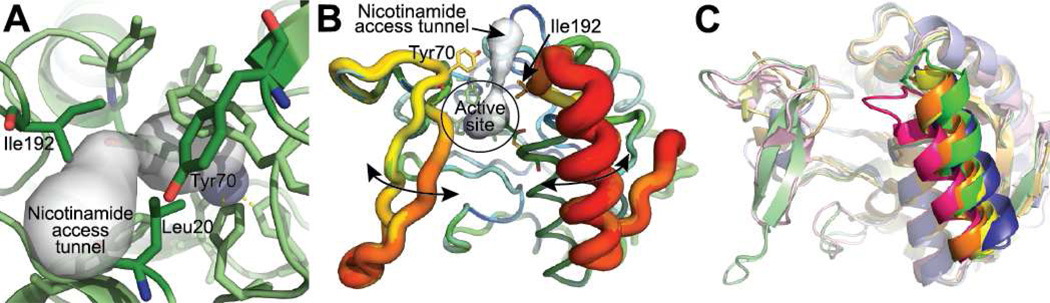
Figure 4.
Putative route of nicotinamide access to the Pnc1 active site. (A) A narrow tunnel exists in the Pnc1 structure from the active site to the protein surface. This tunnel was discovered using Caver (66, 67). (B) The C-terminal loop and helix of the nicotinaldehyde-Pnc1 structure is the most dynamic portion of the protein. All seven Pnc1 monomers in the asymmetric unit were aligned and represented using the b factor putty preset of Pymol (68). The range of b factors in the structure are represented as thin (low b factor) to thick (high b factor) main chains and a color gradient from blue (low b factor) to red (high b factor). (C) Alignment of all published nicotinamidase structures reveals that the C-terminal loop and helix containing I192 is the most dynamic portion of the structures. The nicotinamidases shown are from Pyrococcus horikoshii (PDB 1IM5; yellow), Mycobacterium tuberculosis (PDB 3PL1; orange), Streptococcus pneumoniae (PDB 3O91; blue), Acinetobacter baumannii (PDB 2WT9; pink), and Saccharomyces cerevisiae (this work; green).