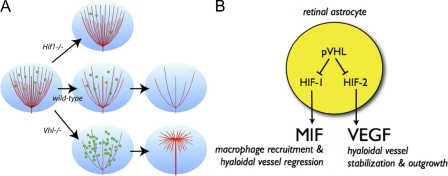
Figure 7.
Current hypothetical model for pVHL-controlled hyaloidal vascular remodeling. (A) Hyaloidal vasculature regression is directed by the oxygen-sensing pVHL/HIF pathway in astrocytes to regulate the flux of infiltrating macrophages. In Hif-1α mutants, Mif levels are attenuated, fewer macrophages are recruited into the vitreous, and hyaloidal vasculature regression is retarded. In Vhl mutants, MIF levels increase dramatically as a result of HIF-1α stabilization; this results in accelerated hyaloidal vasculature regression. Increases in VEGF secretion as a result of HIF-2α stabilization stabilize the stalk of the hyaloidal network and induce secondary outgrowth of vessels along a superficial plane. (B) Astrocytes sense fluctuations in oxygen concentrations in the retina and respond by suppressing pVHL. This in turn stabilizes HIF-1α and HIF-2α that regulate MIF and VEGF, respectively. HIF-1α/MIF signaling regulates macrophage recruitment and controls the rate of hyaloidal vessel regression. Conversely, HIF-2α/VEGF signaling induces pathological neovascularization of hyaloidal vessels when dysregulated.