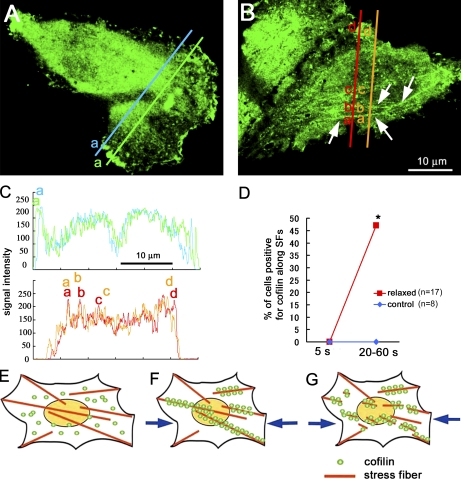
Figure 4.
Cofilin binding to actin stress fibers in intact cells. (A) GFP-cofilin was distributed uniformly in the perinuclear cytosol but was often condensed in the ruffling membrane; GFP-cofilin did not associate with the stress fibers in control cells. (B) When the substratum was relaxed 20%, GFP-cofilin was translocated to actin stress fibers (shown by the arrows: see also Fig. S2) within 1 min. (C) Intensity profiles of the GFP-cofilin along the two lines are shown (the top profile for control cell and the bottom profile for relaxed cell). The broad peak (a) in the top profile corresponds to the cofilin condensed in the ruffling membrane (corresponding with letters in A). The narrow peaks (a, b, and c) in the bottom profile correspond to the stress fibers (corresponding with letters in B). (D) Percentage of cells positive for GFP-cofilin translocated to actin stress fibers increases from 0/17 to 8/17, with relaxing of the substrate (the number of cells examined was 17 from seven independent experiments). Translocation of GFP-cofilin to actin stress fibers (SFs) was not detected in control cells in the same period of observations (five independent experiments). *, P < 0.05, using Fisher’s exact test. (E–G) Schematic drawings of the mechanosensing by the actin stress fibers and the regulation of cofilin binding to actin stress fibers in cells. (E) Actin stress fibers generate contractile force in adherent cells, resulting in generation of tension in stress fibers, which prevents the binding of cofilin to the fibers. (F) When the tension declines (e.g., by relaxing the cell substratum or by decreasing the contractile force in the actin filaments), cofilin binds to and disassembles the fibers (G).