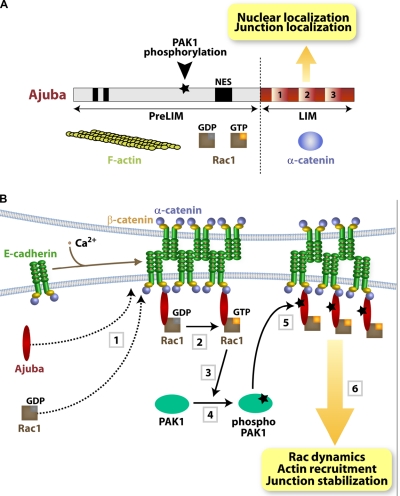
Figure 8.
Summary diagrams. (A) Ajuba domain organization: PreLIM region, three LIM domains, nuclear export signal (NES), and PAK1 phosphorylation site. Protein partners are shown underneath each region. (B) Summary of the data presented. Upon cell–cell contact assembly, Ajuba and Rac are recruited independently to junctions (1). Ajuba interacts with both active (Rac·GTP) and inactive Rac (Rac·GDP) (2). Upon Rac activation by junctions, PAK1 is activated by auto-phosphorylation (3 and 4). Active PAK phosphorylates Ajuba, which increases its affinity to active Rac. Thus, active Rac is stabilized at cell–cell adhesion sites depending on Ajuba phosphorylation levels, thereby fine-tuning locally Rac activation and dynamics (5). The outcome is sustained remodeling of actin filaments and junction stabilization (6). In the absence of Ajuba, Rac can still be recruited and activated by junction assembly, but this is not sufficient to ensure resistance to mechanical stress.