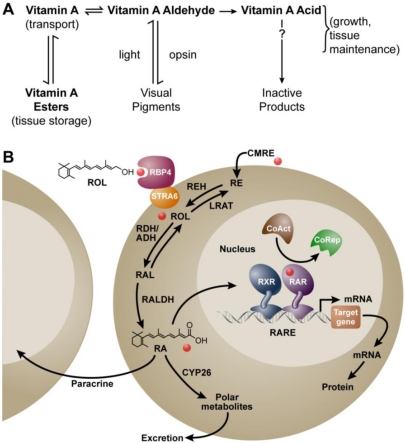
Figure 1.
Metabolism of vitamin A (retinol) to all-trans retinoic acid (RA), and the mechanism of RA action. (A) Metabolic scheme proposed by Dowling and Wald in 1960 [11]; (B) Mechanism ca. 2011. Vitamin A (retinol, ROL) circulates bound to the plasma retinol-binding protein (RBP4) and transthyretin (not shown). RBP4 binds to the membrane receptor STRA6 to facilitate the cellular uptake of retinol in some cells. Vitamin A circulating as part of a chylomicron remnant (CMRE) can also serve as a source of vitamin A for the cell. Note that cellular retinol and RA binding proteins have been omitted for simplicity. Retinol is either esterified by lecithin:retinol acyltransferase (LRAT) and stored, or is oxidized reversibly to retinaldehyde (RAL) by retinol dehydrogenases (RDH/ADH), and further oxidized in irreversible fashion to RA by retinaldehyde dehydrogenase (RALDH 1, 2, or 3). In the nucleus, the RAR/RXR complex is bound to a specific sequence of DNA called the retinoic acid response element (RARE). Binding of RA to the RAR leads to release of the corepressor complex (CoRep) and association with coactivator proteins (CoAct), followed by altered transcription of downstream target genes and ultimately changes in cellular function. RA also undergoes further oxidation by the cytochrome P450 (CYP) 26 family to more polar metabolites. The lipophilic molecule, RA, can act within the same cell in which it is synthesized (autocrine) or can diffuse through the cell membrane to act in nearby cells (paracrine). Abbreviations: ADH, alcohol dehydrogenase; RDH, retinol dehydrogenase; REH, retinyl ester hydrolase; RE, retinyl ester.