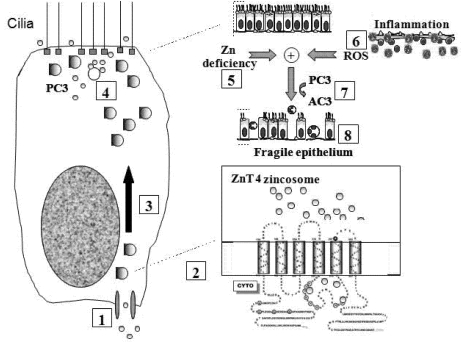
Figure 6.
Model for role of ZnT4 in AE Zn homeostasis and airway inflammation: Step 1: Zn is taken up across the basolateral plasma membrane of AEC from sub-epithelial capillaries via ZIP6 or other ZIP transporter(s); Step 2: Zn is incorporated into vesicles with the aid of ZnT4 (see inset); Step 3: These vesicles translocate to the apical cytoplasm; Step 4: Apical cellular Zn may protect the cytoplasmic mucociliary apparatus (e.g., tubulin and basal bodies) from damage by oxidants and other toxins that would otherwise trigger pro-caspase-3 (PC3) activation. Vesicular Zn may also be secreted across the apical plasma membrane into the epithelial lining fluid, cilia and mucin; Step 5: Apical cellular Zn is depleted in chronic inflammatory airway disease. Mechanisms may include abnormalities in ZnT4, hyper-secretion of Zn or excessive loss of Zn by luminal shedding of dying AEC or exudation of inflammatory cells; Step 6: Depletion of Zn renders AEC vulnerable to reactive oxygen species (ROS) released from inflammatory cells or mitochondria; Step 7: This leads to premature activation of PC3 to active caspase-3 (AC3) and downstream events in apoptosis. Zn depletion also directly facilitates PC3 activation because this enzyme is inhibited by binding of Zn to an essential sulphydryl group [5]; Step 8: The altered epithelium has increased apoptosis and epithelial sloughing, which contribute to the ongoing inflammation.