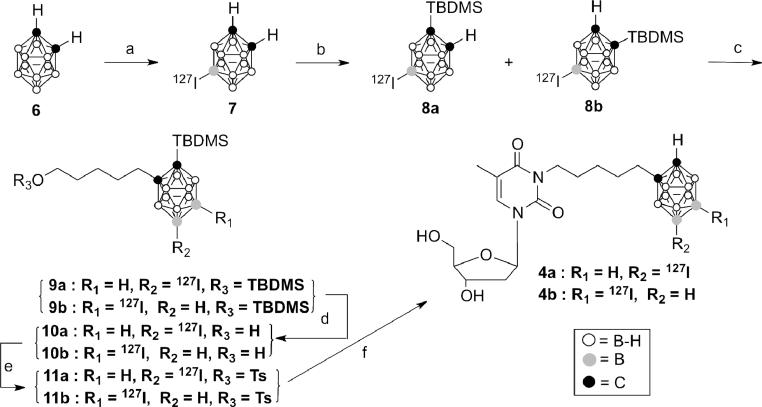
Scheme 2.
Reagents and conditions: a) ICl, DCM, 40°C, 5 h; b) n-BuLi, TBDMSCl, THF, 66°C, 12 h; c) n-BuLi, 5-(tert-butyldimethylsilyloxy)pentyl 4-methylbenzenesulfonate; THF, 66°C, 12 h; d) TBAF, THF, −78°C to 4°C, 30 min; ii) 10% methanolic HCl, 4°C, 30 min; e) TsCl, Et3N, DMAP, 0°C to rt, 7 h; f) dThd, K2CO3, DMF/acetone (1:1), 40°C, 2.5 h.