Abstract
Mounting evidence suggests that helminth infections protect against autoimmune diseases. As helminths cause chronic IgE-mediated activation of basophils and mast cells we hypothesized that continuous activation of these cells could prevents diabetes onset in nonobese diabetic (NOD) mice in the absence of infection. Anti-FcεR1 activated basophils and mast cells and resulted in the release of IL-4 and histamine into the bloodstream. Anti-FcεR1-treated NOD mice showed a type 2 shift in insulin-specific antibody production and exhibited significant delays in diabetes onset. IL-4 responses played a partial role as the protective effect of anti-FcεR1 therapy was diminished in IL-4-deficient NOD mice. In contrast, histamine signaling was not required as anti-FcεR1-mediated protection was not reduced in mice treated with histamine receptor blockers. These results demonstrate that anti-FcεR1 therapy delays diabetes onset in NOD mice and suggest that chronic basophil and mast cell activation may represent a new avenue of therapy for Th1-associated autoimmune diseases.
Keywords: FcεR1, Type 1 diabetes, nonobese diabetic (NOD), IL-4, histamine, IgE
1. Introduction
The prevalence of Type 1 diabetes and other autoimmune diseases has increased sharply over the past few decades [1; 2; 3; 4; 5; 6]. While genetic factors play a role in susceptibility to Type 1 diabetes, it is probable that the dramatic worldwide increase in the prevalence of Type 1 diabetes is due to changes in environmental factors.
One environmental change that may be responsible for the recent increase in autoimmune diseases is the loss of chronic parasitic worm infections in developed countries. Multiple studies have found that individuals infected with chronic parasitic worm infections have lower rates of autoimmune diseases than others living in the same environment (reviewed in [7]. Experimentally, parasitic worms have been shown to protect against Type 1 diabetes and other autoimmune diseases in several animal models [8; 9; 10], and in humans oral administration of porcine whipworm eggs results in protection against inflammatory bowel disease [11].
Until recently, most people had lifelong infections with parasitic worms. As helminths have been identified in neolithic and pre-Columbian mummies [12; 13], it is likely that the human immune system evolved in the setting of chronic infection with these parasites [14]. Consequently, many scientists argue that the loss of parasitic worm infections is partially responsible for the increased prevalence of autoimmune and allergic diseases in developed countries – the notion being that now, in the absence of the immunomodulatory responses triggered by helminths, our immune systems have become hyperresponsive [9; 14; 15].
In this study, we sought to determine whether recapitulation of the IgE-mediated immune responses observed in chronic helminth infections can protect against Type 1 diabetes in the absence of actual infection. Unlike most bacterial or viral pathogens, helminth infections induce the production of specific IgE. This IgE binds to basophils and mast cells through FcεR1, the high affinity IgE receptor. Helminth specific antigens can then activate basophils and mast cells by cross-linking IgE molecules and aggregating FcεR1s. As helminths are large organisms that release substantial amounts of antigen, and as these infections last for years, helminth infections likely induce a state of chronic basophil and mast cell activation. Indeed, recent time course studies in our lab demonstrate that both chronic basophil [16] and chronic mast cell activation (E. Mueller and E. Mitre, unpublished data) occur during infection of mice with the filarial nematode Litomosoides sigmodontis.
There are two mechanistic rationales for postulating that chronic activation of basophils and mast cells may protect against Th1-driven autoimmune disease. First, factors released by basophils and mast cells may have direct immunomodulatory properties that are protective against Th1-mediated autoimmune diseases. Basophils, for example, release large quantities of IL-4 when activated and have been shown to do so in response to parasite antigen in filaria-infected patients [17] as well as in animal models of helminth infection [18; 19; 20; 21]. As destruction of beta-islet cells in Type 1 diabetes is driven by IFNγ release from Th1 cells, and as IL-4 counterregulates Th1 responses and has been shown to improve Th1-driven autoimmune diseases [22; 23], it is possible that chronic basophil activation could protect against Type 1 diabetes by release of IL-4. Similarly, histamine, which is released from both basophils and mast cells, has been shown in vitro to suppress Th1 responses by signaling through the H2 receptor on lymphocytes [24]. Alternatively, chronic activation of basophils and mast cells could induce negative feedback pathways that tamp down ongoing autoimmune responses. Interestingly, there is substantial evidence that chronic immunotherapy, in which patients with IgE-mediated allergies are given weekly injections of allergen, augments immune regulatory networks such as the suppressive cytokine IL-10 and natural T-regulatory cells [25; 26].
To test the hypothesis that chronic activation of basophils and mast cells can protect against a Th1-driven autoimmune disease, we studied the effects repeated anti-FcεR1 antibody injections have on the development of Type 1 diabetes in the NOD mouse model. Our studies demonstrate that anti-FcεR1 therapy activates basophils and mast cells, induces IL-4 and histamine release in vivo, and delays Type 1 diabetes onset in the NOD mouse. Studies using IL-4-deficient NOD mice and therapeutic histamine blockade reveal that IL-4, but not histamine signaling through H1 or H2 receptors, plays a partial role in the protective effects of anti-FcεR1 therapy.
2. Materials and Methods
2.1 Mice
Female NOD and IL-4-deficient NOD.129P2(B6)-Il4tm1Cgn/DvsJ mice (The Jackson Laboratory) were maintained at the Uniformed Services University (USU) animal facility with free access to food and water. All experiments were performed under protocols approved by the USU Institutional Animal Care and Use Committee.
2.2 Assessment of diabetes
Glucose levels of mice were determined from blood taken by orbital bleeds every other week using a standard blood glucose meter (Accu-Check Advantage, Roche Diagnostics GmbH). Mice with glucose levels greater than 230 mg/dl in two consecutive measurements were considered diabetic.
2.3 Treatment with anti-FcεR1
Beginning at 6-weeks of age mice were given weekly i.p. injections of 50 μg Armenian hamster anti-mouse FcεR1 (MAR-1, eBioscience) or control Armenian hamster IgG (Isotype Control, Functional Grade Purified, eBioscience) throughout the experiment.
Subsets of animals were euthanized at 16 weeks of age, one day after the last anti-FcεR1 or IgG injection, to assess pancreas inflammation, splenic and pancreatic lymph node cytokine production, cell subtypes, as well as insulin-specific IgG1 and IgG2c levels.
Different shorter anti-FcεR1 treatment regiments were tested starting at 6-weeks of age. Those regimens included weekly i.p. injections of 50 μg anti-FcεR1 or control IgG for four weeks and i.p. injections of 25 and 50 μg anti-FcεR1 or control IgG twice a week for four weeks.
2.4 Assessment of pancreas inflammation
At 16 weeks of age, pancreases were isolated and fixed in 10% formalin (Protocol, Fisher Scientific Company). Haematoxylin-eosin stained slices were assessed for inflammation by a pathologist (J.T.S.) blinded to the intervention group. Total numbers of islets of three longitudinal sections 400 μm apart of each pancreas were assessed. The severity of insulitis was scored as non-infiltrated, periinsulitis (lymphocytes at the periphery of islets), or intrainsulitis (lymphocyte infiltration into the interior of the islets lesser or greater than 50%).
2.5 Spleen and pancreatic lymph node cell culture
At 16 weeks of age, animals that were treated for 10 weeks with either anti-FcεR1 or control IgG were euthanized and spleen and pancreatic lymph nodes were isolated. Spleen and pancreatic lymph node cells were prepared and cultured as previously reported [8]. In brief, single cell suspensions were obtained, red blood lysis performed for spleen cells (ACK Lysing Buffer, Quality Biological, Inc.), and cells were plated at a concentration of 2×106 cells/ml in enriched media (Iscove's Dulbecco modified medium (Mediatech) including 10% fetal calf serum (Valley Biomedical), 1% L-glutamine (Mediatech), 1% insulin-transferrin-selenium medium (Invitrogen Inc.) and 80 μg/ml gentamicin (Quality Biological, Inc.)), stimulated with 5 μg/ml anti-CD3 and 2 μg/ml anti-CD28 (eBioscience), and cultured at 37°C, 5% CO2.
2.6 Flow cytometric analysis of regulatory cell types and intracellular cytokine production by T cells
Spleen and pancreatic lymph node cells were cultured as described above and prepared for flow analysis as previously reported [8]. In brief, after two hours of incubation, BD GolgiStop was added (BD Biosciences) and cells were incubated for an additional four hours. Collected cells were fixed and permeabilized (eBioscience) overnight. For analysis cells were washed once with phosphate-buffered saline (PBS)/1%BSA (bovine serum albumine, Sigma), followed by a blocking step with PBS/1%BSA. Cells were washed with 1x permeabilization buffer (eBioscience) and stained for five- or four-color-flow using CD4 PerCP, IL-4 APC (BD Biosciences), CD8a Pacific Blue, gamma interferon (IFN-γ) FITC, and IL-17 PE (eBioscience) or CD4 PerCP (BD Biosciences), FoxP3 FITC, CD25 APC-Alexa Fluor 750, and IL-10 PE (eBioscience). For identification of regulatory CD8 T-cells and regulatory B cells, fixed, cryopreserved (PBS/10% DMSO) spleen cells were washed once, blocked with CD16/CD32 (BD Biosciences) and stained with CD4 Qdot 605 (Invitrogen), CD8 PE Cy5, FoxP3 FITC, (all eBioscience) or B220 PerCP, CD1d FITC, CD5 APC (all BD Bioscience), and IL-10 PE (eBioscience). Flow cytometry was performed using a BD LSRII system and subsequently analyzed with FACSDiVa 6.1 software (BD Biosciences).
2.7 Measurement of cytokines and antibodies by ELISA
Cytokine enzyme-linked immunosorbent assays (ELISAs) were performed from spleen and pancreatic lymph node cell cultures. Culture supernatants from cells that were cultured as described above and stimulated with anti-CD3 and anti-CD28 were collected after 72h of incubation. IFN-γ, IL-4, IL-5, and IL-10 were quantified according to the manufacturer's instructions (BD Biosciences).
Plasma derived insulin-specific IgG1 and IgG2c were analyzed by sandwich ELISA as previously described [8]. All samples were analyzed as duplicates at the same time on the same plate to allow accurate comparison between groups by OD.
2.8 Flow cytometric analysis of basophils and mast cells
Frequency of basophils in the peripheral blood and peritoneal mast cells was measured by flow cytometry one day after a single injection of 50 μg anti-FcεR1 or control IgG in 8-week old female NOD mice.
Basophil flow was performed as previously described [16]. In brief, 100 μl of blood obtained by orbital bleeding was diluted with 100 μl of RPMI media (RPMI-1640, Mediatech), red blood cells lysed and cells fixed (whole blood lysing reagent kit, Beckman Coulter, Inc.). After two washing steps with 1x PBS, cells were blocked overnight with PBS/1% BSA. Cells were washed and stained with IgE FITC (BD Biosciences), CD4 PerCP (BD Biosciences) and B220 PerCP (BD Biosciences). During flow cytometric analysis, basophils were identified as CD4-/B220-/IgE+ cells.
Mast cells were obtained by a peritoneal wash with 1x HBSS (Gibco) and fixed after red blood cell lysis (whole blood lysing reagent kit, Beckman Coulter). Cells were washed once and blocked overnight in PBS/1% BSA. 1×106 peritoneal cells were stained for flow with IgE FITC (BD Biosciences) and cKit APC (BD Biosciences). During flow cytometric analysis, mast cells were identified as IgE+/cKit+ cells.
2.9 Detection of histamine from in vitro stimulated purified mast cells
For in vitro activation of mast cells, peritoneal mast cells were purified using a 70% percoll gradient. 2000 mast cells were cultured in 250 μl histamine release buffer (Immunotech S.A.S.) and stimulated with isotype (10 μg/ml) or anti-FcεR1 (0.4, 1.6, 6.25, 25, 100 μg/ml) for 30 minutes. Cells were acylated and histamine ELISA performed according to the manufacturer's instructions (Immunotech S.A.S.).
2.10 Intracellular IL-4 detection of in vitro stimulated basophils
For intracellular IL-4 detection of basophils, 100 μl of peripheral blood from NOD mice was cultured with GolgiStop (BD Bioscience) and either media, 10 μg/ml isotype control, 10 or 25 μg/ml anti-FcεR1 (MAR-1) for a total of 6h at 37°C. Following red blood lysis and fixation (performed as mentioned above) cells were stained in a two step procedure for flow. Cells were stained for the surface markers IgE FITC, CD4 PerCP, and B220, followed by permeabilization (permeabilization buffer, BD Bioscience) and staining with IL-4 APC (BD Bioscience).
As basophils are the only circulating cells in mice that express FcεR1, IL-4 release from whole blood was measured after in vitro stimulation of 100 μl of peripheral blood from NOD mice cultured with 10 μg/ml anti-FcεR1 antibody, 10 μg/ml IgG isotype control, or media for 6h at 37°C. Plasma was obtained by centrifugation and stored at -20°C until the IL-4 ELISA was performed as per the manufacturer's instructions (eBioscience).
2.11 In vivo cytokine capture assay to detect IL-4 release after anti-FcεR1 injection
10 μg biotinylated IL-4 antibody (BD Bioscience) was injected i.p. into 11-week old NOD mice. The following day mice were injected i.p. with 50 μg anti-FcεR1 or control IgG and one day later blood was collected by orbital bleed. Concentration of IL-4 was measured by ELISA according to the manufacturer's instructions (BD Biosciences).
2.12 Detection of in vivo histamine release after injection of anti-FcεR1
Mice were i.p. injected with a single dose of 50 μg anti-FcεR1 or control IgG. After 30 minutes, blood was collected by orbital bleed. 100 μl of blood were immediately acylated and histamine ELISA was performed according to the manufacturer's instructions (Immunotech S.A.S.).
2.13 Treatment with histamine receptor blockers
Starting at 6-weeks of age, 2.5 mg/ml cimetidine (Sigma), an H2-receptor blocker, and 0.25 mg/ml fexofenidine HCl (TEVA Pharmaceuticals USA), an H1-receptor blocker, were added to the drinking water of NOD mice. The treated drinking water was freshly prepared every other day throughout the experiment until 25 weeks of age.
Efficacy of H2-receptor blockage was tested by measuring the stomach pH 2 days after changing the drinking water in mice that had been receiving fexofenidine and cimetidine treatments for 2 weeks. Mice were euthanized and the stomach pH was measured using pH strips (Micro Essential Laboratory). In addition, blockage of the histamine-receptors was confirmed in vivo by injecting 10 μg anti-FcεR1 intradermally into one ear and 10 μg control IgG in the other, followed by i.v. injection of 200 μl toludine blue (Sigma, 0.5% in PBS) 3 minutes later. The ears were dissected 10 min later and vascular permeablility was measured at 620 nm by the optical density (OD) of the blue dye extracted from the ears after o.n. incubation at 63°C in Formamide (Sigma).
2.14 Statistics
Statistical analyses were performed with GraphPad Prism software (GraphPad Software). Differences between paired groups for in vitro studies were analyzed using one-tailed paired T-test and differences between two unpaired groups for in vivo studies were tested for significance with the Mann-Whitney-U-test. P-values <0.05 were considered significant. All experiments were performed at least twice.
3. Results
3.1 Anti-FcεR1 antibody activates basophils and mast cells of NOD mice
To evaluate the effects anti-FcεR1 antibody has on mast cells in vitro, histamine release from mast cells purified from the peritoneal cavity of NOD mice was assessed in response to in vitro incubation with various concentrations of anti-FcεR1. Density gradient centrifugation of peritoneal cells resulted in high level enrichment of mast cells (>70% purity for all samples, Fig 1A). While incubation of enriched mast cells with media or isotype antibody resulted in no demonstrable histamine release above incubation with media alone, incubation with increasing concentrations of anti-FcεR1 antibody resulted in a classical mast cell activation curve (Fig 1B).
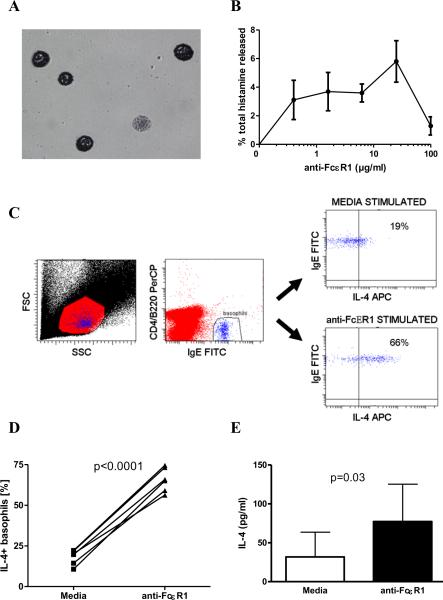
Figure 1. Anti-FcεR1 activates basophils and mast cells in vitro.
(A) May-Grünwald staining of peritoneal mast cells after enrichment by density centrifugation. (B) Histamine release from mast cells in response to increasing concentrations of anti-FcεR1. (C) Representative dot plots of intracellular IL-4 staining of basophils after incubation with media or anti-FcεR1. Initial gating of lymphocytes and lower half of granulocyte region in FSC/SSC plot (left panel). Basophils identified as CD4-B220-IgE+ (middle panel). IL-4 staining of basophils after incubation with media (upper right panel) or anti-FcεR1 (lower right panel). (D) Percentages of basophils that stain positively for IL-4 after incubation with media or anti-FcεR1. (E) IL-4 release from whole blood after incubation with anti-FcεR1. Error bars denote SEM. Results from 4-6 animals per group are shown. Each experiment was independently performed twice. Statistical significance assessed per one-tailed paired T-test.
To determine whether anti-FcεR1 antibody also activates basophils in vitro, whole blood samples from NOD mice were assessed for basophil IL-4 production by intracellular flow cytometry. Basophils were identified by flow cytometry as CD4-B220-IgE+ cells (Fig. 1C) as this staining strategy has been shown to be specific for basophils [16]. Whereas an average of 18% of basophils stained positively for IL-4 at baseline, on average 65% were IL-4+ after incubation with anti-FcεR1 (p<0.0001, Fig 1D). To confirm that anti-FcεR1 induces IL-4 release from basophils, IL-4 was measured by ELISA in the supernatants of whole blood samples incubated with media or anti-FcεR1. As seen in Figure 1E, anti-FcεR1 caused significant release of IL-4 from whole blood (unstimulated mean IL-4 concentration 32 pg/ml vs. mean 77 pg/ml after anti-FcεR1 stimulation, p=0.03). As basophils are the only circulating cells in mice that express FcεR1, this assay confirms that anti-FcεR1 antibody induces IL-4 release from basophils.
3.2 Anti-FcεR1 injections deplete basophils and induce massive histamine and IL-4 release in vivo
As both basophils and mast cells express the FcεRI on their surface, we tested whether treatment with anti-FcεR1 antibody depletes either peripheral basophils or peritoneal mast cells by injecting NOD mice with 50 μg of anti-FcεR1 and measuring blood basophil and peritoneal mast cell frequencies by flow cytometry. The frequency of blood cells that were basophils was significantly reduced one day after treatment with anti-FcεR1 antibody compared to control animals (median 0.11%, range 0.05-0.19% vs. 0.40%, 0.19-0.63%, Fig. 2A) whereas the frequency of peritoneal mast cells was unchanged (median 1.8%, range 0.2-2.5% vs. 2.1%, 0.1-3.2%, Fig. 2B). This finding is consistent with that of other research groups who have used anti-FcεR1 antibody to deplete mice of basophils [21; 27].
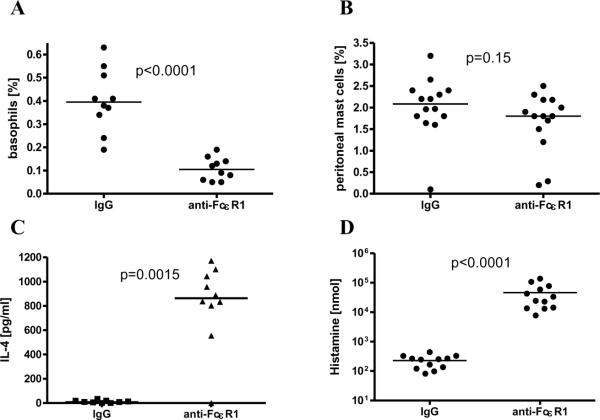
Figure 2. Effects of anti-FcεR1 injections in vivo.
Percentages of circulating white blood cells that are basophils (A) and percentages of peritoneal cells that are mast cells (B) in mice one day after intraperitoneal injection of 50 μg of isotype control antibody or anti-FcεR1. (C) Plasma IL-4 concentration as measured by in vivo cytokine capture assay one day after anti-FcεR1 or isotype antibody injection. (D) Histamine concentration in plasma 30 minutes after anti-FcεR1 or isotype antibody injection. Statistical significance between groups was assessed by the Mann-Whitney test (*<0.05). Joined data from two independent experiments is shown with 5 animals per group and experiment.
Because aggregation of surface FcεR1 activates basophils and mast cells, and since our in vitro studies demonstrated that anti-FcεR1 antibody induces basophils and mast cells to release IL-4 and histamine, we tested whether injection of anti-FcεR1 antibody increases blood levels of IL-4 and histamine. As seen in Figure 2C, mice that were injected the previous day with anti-FcεR1 had significantly higher frequencies of circulating IL-4, as measured by in vivo cytokine capture assay, compared to isotype antibody treated controls (p=0.0015, median 863 pg/ml, range 0-1173 pg/ml vs. 10 pg/ml, range 0-34 pg/ml). Similarly, histamine, which was measured in peripheral blood obtained 30 minutes after antibody injection due to its very short half-life, was substantially higher in anti-FcεR1-treated mice than in controls. Mice that received anti-FcεR1 had a median blood histamine concentration of 28800 nmol (range 7745-137700 nmol) as compared to only 248 nmol (range 81-441 nmol, p<0.0001, Fig. 2D). As basophils and mast cells are the only cells that contain pre-formed histamine, these results demonstrate that anti-FcεR1 antibody injection activates basophils and mast cells in vivo.
3.3 Anti-FcεR1 treatment delays diabetes onset in NOD mice
To test the hypothesis that chronic activation of basophils and mast cells protects against autoimmune diseases, development of Type I diabetes was monitored in NOD mice that were given repeated anti-FcεRI antibody injections.
Continuous injections with anti-FcεR1 significantly reduced blood glucose concentrations in NOD mice at 20 and 24-28 weeks of age compared to control animals (Fig. 3A) and delayed the onset of diabetes (onset of diabetes in 50% of the animals: IgG group: 20 weeks of age, anti-FcεR1: 30 weeks of age, Fig. 3B). Histological analysis of the pancreas revealed that anti-FcεR1-treated mice had an increased total number of β-islet cells and less lymphocyte infiltration of the pancreatic islets compared to IgG treated mice at 16-weeks of age, although these differences were not statistically significant (Fig. 3C).
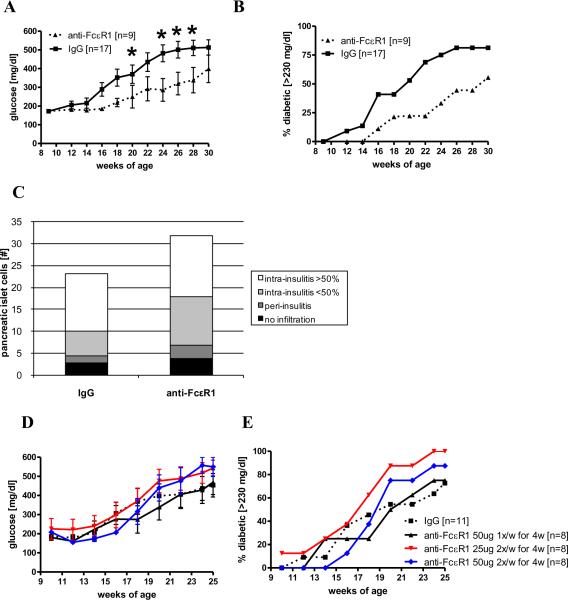
Figure 3. Treatment with anti-FcεR1 delays the onset of diabetes.
(A) Mean blood glucose levels and (B) percentages of NOD mice with diabetes during treatment with weekly injections of 50 μg anti-FcεR1 (n=9) or IgG control antibody (n=17). (C) Mean total numbers of pancreatic islets from anti-FcεR1 or isotype treated mice at 16 weeks of age (9-10 animals per group). Pancreatic islets were classified as non-infiltrated, periinsulitis, and intrainsulitis with less than or more than 50% infiltrated lymphocytes. (D) Blood glucose levels and (E) percentages of diabetic mice that received either weekly injections of 50 μg anti-FcεR1 for four weeks or injections of 25 or 50 μg anti-FcεR1 twice a week for four weeks (n=8 per group) or isotype controls (n=11). Error bars denote SEM. Statistical significance between groups was assessed by the Mann-Whitney test (*<0.05). Shown are the results from 2-3 independent experiments.
Shorter anti-FcεR1 antibody treatment periods from 6-9 weeks of age did not provide a similar protective effect. Neither 4 weekly injections with 50 μg of anti-FcεR1 antibody, nor 4 weeks of injections with 25 or 50 μg twice a week decreased blood glucose levels or the frequency of diabetic NOD mice compared to isotype antibody-treated controls (Fig. 3D, E).
3.4 Anti-FcεR1 treatment induces a Th2 shift in antibody, but not cytokine production
The effect 10 weeks of anti-FcεR1 treatment has on cellular cytokine production was evaluated from spleen and pancreatic lymph node cells of 16 week old NOD mice after stimulation with anti-CD3/anti-CD28. IFNγ and IL-4 levels were similar between isotype antibody-injected controls and anti-FcεR1-treated animals (Fig. 4A, B), whereas the concentration of IL-5 was significantly increased in anti-FcεR1-treated mice (Fig. 4C). Pancreatic lymph node cells from anti-FcεR1-injected mice produced more IL-5 and IFNγ, in response to anti-CD3/anti-CD28 compared to controls, although these differences did not reach statistical significance (Fig. 4D, E).
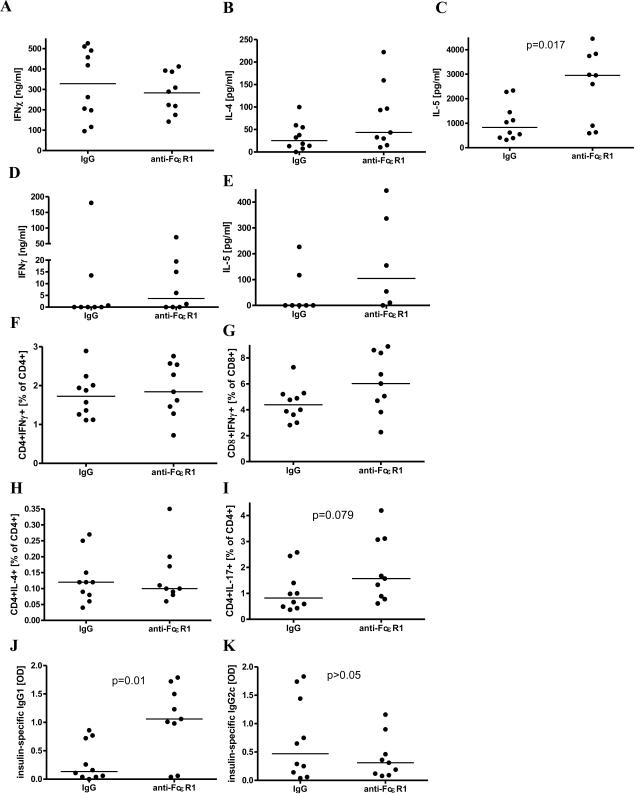
Figure 4. Cytokine and antibody response after 10 weeks of anti-FcεR1/isotype treatment at 16 weeks of age.
(A) IFNγ, (B) IL-4, and (C) IL-5 cytokine production from spleen cells and (D) IFNγ and (E) IL-5 cytokine production from pancreatic lymph node cells in response to anti-CD3/anti-CD28. (F) Frequency of splenic CD4+IFNγ+ and (G) CD8+IFNγ+, as well as (H) CD4+IL-4+ and (I) CD4+IL-17+ T cells in response to anti-CD3/anti-CD28. (J) Plasma levels of insulin-specific IgG1 and (K) IgG2c. Statistical significance between groups was assessed by the Mann-Whitney test. Shown are the results from two independent experiments with a total of 9-10 animals per group.
Frequencies of splenic CD4+IFNγ+ T cells and CD8+IFNγ+ T-cells (Fig. 4F, G) as well as CD4+IL-4+ T-cells (Fig. 4H) after in vitro stimulation with anti-CD3/anti-CD28 were not significantly affected by anti-FcεR1 treatment. Frequencies of splenic anti-CD3/anti-CD28 stimulated CD4+IL-17+ T-cells were increased in anti-FcεR1-treated animals compared to controls, though this difference did not reach statistical significance (p=0.079, Fig. 4I).
As antigen-specific cellular immune responses are difficult to detect in NOD mice, we tested whether repeated anti-FcεR1 antibody injections induce either insulin-specific Th1 (IgG2c) or Th2 (IgG1) associated antibody subsets. Mice that were treated for 10 weeks with anti-FcεR1 antibodies had significantly increased levels of insulin-specific IgG1 compared to isotype antibody-injected controls whereas levels of insulin-specific IgG2c were unchanged (Fig. 4J, K).
3.5 Anti-FcεR1 treatment induces a marginal increase in regulatory responses
Frequencies of regulatory T and B cells and the production of the immunoregulatory cytokine IL-10 were measured from spleen and pancreatic lymph node cells in response to anti-CD3/anti-CD28 after 10 weeks of anti-FcεR1 antibody treatment to assess the development of a regulatory immune response.
While repeated anti-FcεR1 antibody treatment did not substantially alter the percentage of CD4+T-cells that were CD25+FoxP3+ in the spleen (mean 12.5% in isotype treated mice vs. 13.3% in anti-IgE-treated), mean percentages of CD4+CD25+FoxP3+ regulatory T cells in the pancreatic lymph nodes did increase substantially (3% in isotype-treated vs. 5.2% in anti- FcεR1-treated, Fig 5B) though this difference did not reach statistical significance (p=0.17).
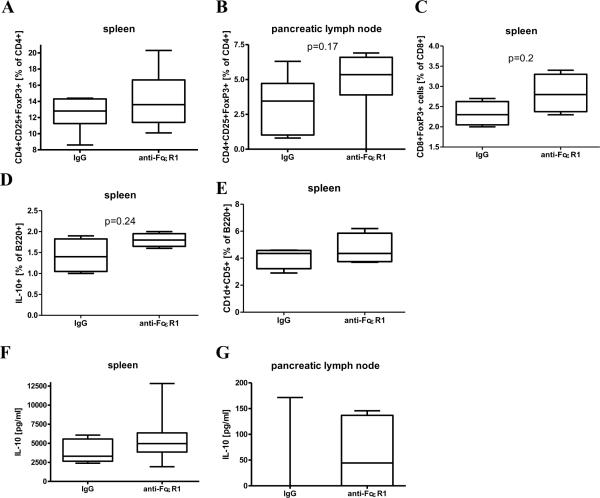
Figure 5. Frequency of regulatory cell types and IL-10 production after 10 weeks of anti-FcεR1/isotype treatment at 16 weeks of age.
(A) Frequency of splenic and (B) pancreatic lymph node CD4+CD25+FoxP3+ regulatory T cells in response to anti-CD3/anti-CD28. (C) Frequency of spontaneous splenic CD8+FoxP3+ regulatory T cells, (D) anti-CD3/anti-CD28 stimulated IL-10+ B-cells, and (E) B220+CD5+CD1d+ regulatory B-cells. (F) IL-10 release from spleen as well as (G) pancreatic lymph node cells in response to anti-CD3/anti-CD28. Statistical significance between groups was assessed by the Mann-Whitney test (spleen: 9-10 animals per group, pancreatic lymph node: 6 animals per group, CD8+ and B-regulatory cells: 4 animals per group). Shown are the results from two independent experiments.
Similarly, percentages of CD8+ spleen cells that were positive for FoxP3+ (Fig. 5C), splenic B-cells that produced IL-10 (Fig. 5D), and splenic B-cells that expressed a regulatory phenotype (B220+CD1d+CD5+, Fig. 5E) were all increased after anti-FcεR1-treatment, though none reached statistical significance.
Repeated anti-FcεR1 antibody injections also slightly increased splenic (p=0.18, Fig. 5F) and pancreatic lymph node cell (p=0.44, Fig. 5G) IL-10 production in response to anti-CD3/anti-CD28, though again the increases were not statistically significant.
3.6 Histamine signaling through H1 and H2 receptors is not necessary for anti-FcεR1 induced delay of Type I diabetes
As injection with anti-FcεR1 resulted in the release of increased amounts of histamine and as histamine can have immunomodulatory properties, we tested whether blockade of H1 and H2 histamine receptors would prevent anti-FcεR1-mediated delay of diabetes onset in NOD mice.
Cimetidine (an H2 receptor blocker) and fexofenadine (an H1 receptor blocker) were administered by addition to the drinking water of NOD mice from 6 weeks of age to study endpoint at 25 weeks of age at concentrations used by prior investigators [28; 29].
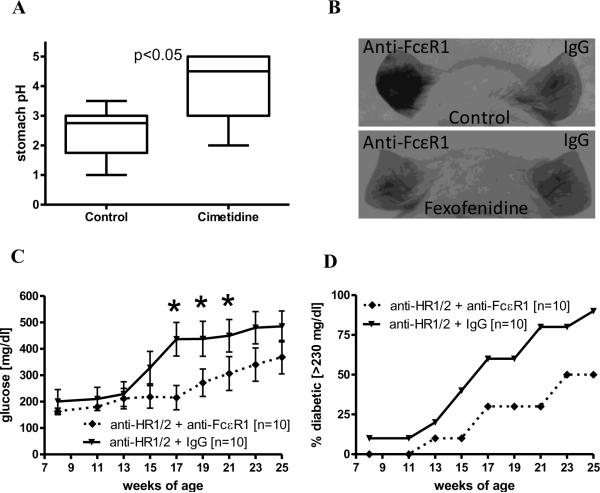
Efficacy of H2-receptor blockade was confirmed by a significant increase in the stomach pH of treated animals compared to controls (Fig. 6A). Blockade of H1-receptors was confirmed by evaluating changes in vascular leak in response to intradermal anti-FcεR1 injection. To improve visualization of vascular leakage in the ear, NOD mice were given intravenous injections of toluidine blue. Control NOD mice exhibited substantial local vascular leakage in response to intradermal injection with anti-FcεR1 antibody whereas fexofenadine-treated mice did not (Fig 6B, left ears). Vascular leakage was a specific response to anti-FcεR1 antibody, as intradermal injection of control antibody did not induce vascular leakage (Fig 6B, right ears).
Figure 6. Histamine receptor blockage does not decrease anti-FcεR1 mediated delay of diabetes onset.
(A) Stomach pH from animals that were treated for 2 weeks with the histamine receptor blockers cimetidine and fexofenidine (5 animals per group). (B) Representative pictures of mice that were injected i.v. with toluidine blue following challenge with anti-FcεR1 (left ear) and IgG control (right ear). The bottom mouse was treated with fexofenidine, a histamine receptor 1 blocker, whereas the top mouse is an untreated control. (C) Blood glucose levels and (D) percentages of NOD mice with diabetes in the setting of continuous administration of histamine receptor blockers (anti-HR1/2) and treatment with weekly injections of 50 μg anti-FcεR1 or IgG control antibody. Shown are the results from two independent experiments with a total of 10 mice per group. Error bars denote SEM. Statistical significance between groups was assessed by the Mann-Whitney test (*<0.05).
As seen in figures 6C and 6D, continuous histamine receptor blockade did not negate the protective effect of anti-FcεR1 antibody treatment on the development of diabetes in NOD mice. Anti-FcεR1 antibody treatment during histamine receptor blockade resulted in significantly reduced blood glucose levels at 17-21 weeks of age (Fig. 6C) and delayed the onset of diabetes compared to control animals (onset of diabetes in 50% of the animals: IgG isotype group: 17 weeks of age, anti-FcεR1 antibody group: 23 weeks of age, Fig. 6D).
3.7 Efficacy of anti-FcεR1 antibody-mediated protection is reduced in the absence of IL-4
As anti-FcεR1 antibody injection increased in vivo IL-4 levels and as IL-4 can counterbalance pathogenic Th1 autoimmune responses, we tested whether the protective effect of anti-FcεR1 antibody was dependent on IL-4 by treating IL-4-deficient NOD mice with repeated weekly anti-FcεR1 injections.
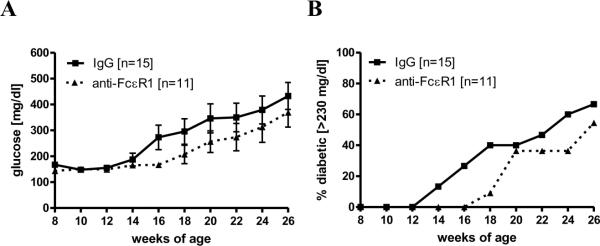
Weekly injections of anti-FcεR1 or control IgG were given from 6-20 weeks of age. Although blood glucose levels were reduced in anti-FcεR1-treated IL-4-deficient animals (Fig. 7A) the difference compared to IgG treated animals was less prominent than had been previously observed in immunocompetent NOD mice (Fig 3A) and did not reach statistical significance. Similarly, protection against overt diabetes, while still present (onset of diabetes in 50% of the animals: isotype antibody group: 24 weeks of age, anti- FcεR1 antibody group: 26 weeks of age, Fig. 7B), was substantially less than had been observed in immunocompetent mice (Fig 3B).
Figure 7. Deficiency of IL-4 reduces anti-FcεR1 mediated protective effect.
(A) Blood glucose levels and (B) percentages of mice with diabetes in IL-4 deficient NOD mice that were injected weekly with 50 μg anti-FcεR1 (n=11) or an IgG control antibody (n=15). Error bars denote SEM. Statistical significance between groups was assessed by the Mann-Whitney test (*<0.05). Shown are the results from four independent experiments.
4. Discussion
This study demonstrates that repeated anti-FcεR1 therapy protects against Type 1 diabetes in the NOD mouse model. Continuous administration of anti-FcεR1 antibody reduced the incidence of diabetes in NOD mice by ~50% compared to controls and was associated with increased total numbers of pancreatic β-islet cells. The protective effects appeared dependent on chronic treatment with anti-FcεR1 antibody, as shorter treatment regimens for 4 weeks did not result in appreciable disease protection. Future studies will optimize anti-FcεR1 antibody concentrations and administration intervals to further improve protection.
In vitro and in vivo assays showed that anti-FcεR1 antibody activates basophils and mast cells and greatly increases circulating levels of histamine and IL-4. This result was not unexpected, as basophils and mast cells both release histamine and IL-4 in response to FcεR1-mediated signaling. We suspect that the bulk of the IL-4 release was due to basophil activation, since basophils are the only cells known to store large quantities of pre-formed IL-4 [30], are major contributors of IL-4 in helminth infections and allergic diseases [17; 19; 31], and have been demonstrated to release more IL-4 on a per cell basis than other cell types [32; 33]. To our knowledge this is the first demonstration that chronic activation of basophils and mast cells is associated with protection against autoimmunity.
Although continuous treatment with anti-FcεR1 antibody did not result in a clear Th2 shift in cytokine production, as measured from spleen and pancreatic lymph node cells, increases in insulin-specific IgG1 revealed a type 2 immune shift in antibody production. Increased serum levels of IL-4 following anti-FcεR1 antibody treatment suggest that this type 2 immune shift in antibody responses may have been driven by IL-4 released from innate cells activated by antibody rather than T-cells. This type 2 immune shift in insulin-specific antibody isotypes is similar to that observed when NOD mice are infected with the filarial nematode Litomosoides sigmodontis [8], an intervention that also results in protection against diabetes.
While histamine signaling through H1 and H2 receptors does not appear necessary for protection, as treatment with H1 and H2 receptor blockers did not attenuate the protective effects of anti-FcεR1 injections, results obtained with IL-4 deficient NOD mice demonstrate that IL-4 is partly responsible for therapeutic benefit. The finding that IL-4 protects against autoimmune disease is consistent with prior studies. In NOD mice systemic administration of IL-4 [34; 35], expression of IL-4 by pancreatic β-islet cells [36], and transfer of IL-4 expressing DCs [37; 38] have been shown to prevent the onset of autoimmune diabetes. An important role for IL-4 in the control of Th1-driven autoimmune diseases is further suggested by studies that used helminths to prevent autoimmunity. Helminth or helminth antigen induced protection against autoimmune diabetes is associated with the induction of Th2 immune responses [8] and studies in experimental autoimmune encephalitis and trinitrobenzene sulfonic acid (TNBS) induced colitis showed that Schistosoma egg administration failed to protect against autoimmunity in mice deficient in STAT6 or depleted of IL-4 [39].
Besides counterbalancing Th1 immune responses, FcεR1-induced IL-4 may protect against Th1 driven autoimmune responses by driving the differentiation of classically (Th1 associated) macrophages into an alternative activated phenotype (AAMØ). AAMØ are anti-inflammatory and are known to be more prevalent during helminth infections [40]. Future studies will investigate whether FcεR1-treatment induces AAMØ and whether this cell population contributes to the protective effect.
The novelty of this study is that we were able to induce IL-4 release by systemically activating basophils and mast cells with an antibody that directly cross-links FcεR1s. An approach that is based on antibody injections to trigger IL-4 release might be easier to transfer to the bedside compared to therapies that consist of injection with cytokines.
Additionally, given that the protective effects of anti-FcεR1 therapy were only partially reduced in IL-4-deficient NOD mice, it is likely that IL-4 independent mechanisms may also play a role in anti-FcεR1 therapy. These may be directly related to activation of basophils and/or mast cells, or they may be due to induction of negative feedback pathways induced by chronic activation of these cells. Determining the protective mechanisms of repeated anti-FcεR1 injections that are IL-4 independent will be a focus of studies in the future.
One IL-4-independent mechanism may be the induction of IL-13 release by anti-FcεR1 injections as IL-13 has been shown to prevent diabetes onset in NOD mice [41]. Both basophils as well as mast cells can produce IL-13 after cross linking of FcεR1. Because IL-13 signals through IL-4Rα it is possible that anti-FcεR1-induced IL-13 assumed some of the functions of IL-4 in IL-4 deficient NOD mice and contributed to anti-FcεR1 mediated protection. Evaluating whether IL-13 plays a role in anti-FcεR1 mediated protection against autoimmunity will be the subject of future studies.
In contrast, the observed non-signficant increase of Th17 cells during anti-FcεR1 therapy is very unlikely to be a mechanism by which anti-FcεR1 injections protect against Type 1 diabetes since Th17 responses are thought to have a role in the induction of Type I diabetes, possibly by conversion of Th17 to Th1 cells that can cause diabetes onset [42; 43; 44; 45].
Another IL-4-independent mechanism that may be important is the induction of immunoregulatory networks. By repeatedly activating basophils and mast cells, we replicated the immunological phenotype observed in chronic helminth infections and in allergen immunotherapy. In helminth infections, basophils and mast cells are continuously being activated by parasite antigens through parasite-specific IgE on the surface of these cells [46]. In immunotherapy, patients with allergen-specific IgE are repeatedly given injections of allergen to which they have specific IgE, essentially inducing a chronic state of low level basophil and mast cell activation. While the mechanisms by which chronic helminth infections and allergen immunotherapy modulate the immune system are not completely understood, a number of studies show that both augment key regulators of peripheral tolerance such as IL-10 and T-regulatory cells. Indeed, helminth infections shown to protect against autoimmune diseases in animal models have been repeatedly associated with increases of IL-10 and T-regulatory cells [8; 47; 48].
In our study, while not statistically significant, levels of IL-10 production from splenocytes, frequencies of IL-10 producing B cells, and frequencies of regulatory CD4+FoxP3+ T-cells, CD8+FoxP3+ T-cells, and CD1d+CD5+ B-cells were all higher in mice receiving anti-FcεR1 antibody injections. As one of the hallmarks of autoimmune diseases is the loss of peripheral tolerance [49], it will be important for future studies to more fully evaluate whether anti-FcεR1 therapy functions by augmenting peripheral tolerance. In a similar vein, it would be interesting to evaluate whether allergen immunotherapy in allergic patients has had beneficial effects on patients with concurrent autoimmune diseases.
Future studies will also attempt to further define exactly which cells are involved in anti-FcεR1-mediated protection. In particular, it will be important to determine whether basophils and/or mast cells are necessary for the protective effects of anti-FcεR1 therapy. We have shown that anti-FcεR1 therapy activates these cells, but have not yet demonstrated that these cells are essential. As there are currently no basophil-deficient nor mast-cell deficient mice on a NOD background, it will be necessary to develop techniques of depleting basophils and mast cells without concurrently activating them. Additionally, it has recently been shown that antigen-presenting DCs can express FcεR1 in the setting of Th2 inflammation and be depleted by anti-FCεR1 treatment (15). While it is unlikely these cells play a role in the Th1-mediated pathology of Type 1 diabetes, future studies will also evaluate whether FcεR1+ DCs play a role in FcεR1-mediated protection against Type I diabetes.
Clinically, it is important to ask whether induction of chronic basophil and mast cell activation could actually be used to protect against autoimmune diseases. While at first blush such an approach sounds preposterous given the obvious risk of anaphylaxis, we believe chronic basophil and mast cell activation could be a safe and feasible therapy. We propose that the key to safety would be to start treatment with small amounts of anti-FcεR1 antibody, or another basophil/mast cell activating agent, followed by a gradual increase in treatment dosage. Repeated administration of gradual increasing amounts of anti-FcεR1 did not cause anaphylaxis in the mice we studied, and such an approach would be similar to allergen immunotherapy, in which the dose of allergen given is gradually increased over time. Allergen immunotherapy induces repeated basophil and mast cell activation and is routinely conducted in the outpatient arena for diseases as benign as allergic rhinitis.
Importantly, unlike conventional therapies for autoimmune diseases which predominantly work by incapacitating specific arms of the immune system, a therapeutic approach based on the induction of chronic basophil and mast cell activation has the potential to induce a therapeutic effect without irreversibly inhibiting any pathways of the immune system.
In conclusion, this study demonstrates that repeated administration of anti-FcεR1 antibodies results in activation of basophils and mast cells and protection against Type 1 diabetes in NOD mice by a mechanism that is partially dependent on IL-4. While IL-4 has been previously shown to protect against Th1-driven autoimmune diseases, the utilization of repeated antibody injections to induce IL-4 release represents a novel approach for the treatment of autoimmune diseases. Given the feasibility of developing antibodies for use in humans, such an approach has the potential to be translated into a novel clinical therapy for Th1-driven autoimmune diseases. In addition to evaluating the safety and feasibility of this approach as a therapy for humans with autoimmune diseases, future studies will also focus on obtaining a better understanding of how helminth infections protect against autoimmune diseases to enable development of other novel therapeutic strategies.
Highlights.
anti-FcεR1 treatment activates basophils and mast cells and induces IL-4 and histamine release in vivo
anti-FcεR1 treatment delays the onset of Type I diabetes in NOD mice
the protective effect of anti-FcεR1 therapy is partially dependent on IL-4 and is independent of histamine signaling through H1 or H2 receptors
Acknowledgments
We thank Dr. J. Thomas Stocker from the Dept. of Pathology at USU for his help to assess inflammation in the pancreas and Dr. Kristin Tarbell from NIH/NIDDK for helpful discussions on working with the NOD model of diabetes.
This work was supported by grants 1DP2DK083131 from NIH/ NIDDK and R073MX from the Uniformed Services University of the Health Sciences.
Drs. Mitre and Hübner are co-inventors on U.S. Provisional Patent Application No. 61/382,541 “Chronic basophil activation as a therapy for Th1-driven autoimmune diseases”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Current address: Marc P Hübner joined the Institute for Medical Microbiology, Immunology and Parasitology, University Hospital Bonn, Sigmund-Freud-Str. 25, Building 320, 53105 Bonn, Germany.
References
- 1.Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: A systematic review of international trends. Inflamm Bowel Dis. 2010 doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 2.Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51:3353–61. doi: 10.2337/diabetes.51.12.3353. [DOI] [PubMed] [Google Scholar]
- 3.Koutsouraki E, Costa V, Baloyannis S. Epidemiology of multiple sclerosis in Europe: a review. Int Rev Psychiatry. 2010;22:2–13. doi: 10.3109/09540261003589216. [DOI] [PubMed] [Google Scholar]
- 4.Loftus EV., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 5.Logan I, Bowlus CL. The geoepidemiology of autoimmune intestinal diseases. Autoimmun Rev. 2010;9:A372–8. doi: 10.1016/j.autrev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes--the analysis of the data on published incidence trends. Diabetologia. 1999;42:1395–403. doi: 10.1007/s001250051309. [DOI] [PubMed] [Google Scholar]
- 7.Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune-mediated inflammation. Int J Parasitol. 2007;37:457–64. doi: 10.1016/j.ijpara.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Hübner MP, Stocker JT, Mitre E. Inhibition of type 1 diabetes in filaria-infected non-obese diabetic mice is associated with a T helper type 2 shift and induction of FoxP3+ regulatory T cells. Immunology. 2009;127:512–22. doi: 10.1111/j.1365-2567.2008.02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaccone P, Burton OT, Cooke A. Interplay of parasite-driven immune responses and autoimmunity. Trends Parasitol. 2008;24:35–42. doi: 10.1016/j.pt.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–13. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 11.Summers RW, Elliott DE, Urban JF, Jr., Thompson R, Weinstock JV. Trichuris suis therapy in Crohn's disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allison MJ, Pezzia A, Hasegawa I, Gerszten E. A case of hookworm infestation in a Precolumbian American. Am J Phys Anthropol. 1974;41:103–6. doi: 10.1002/ajpa.1330410113. [DOI] [PubMed] [Google Scholar]
- 13.Aspöck H, Auer H, Picher O. Trichuris trichiura eggs in the neolithic glacier mummy from the Alps. Parasitology Today. 1996;12:255–256. [Google Scholar]
- 14.Jackson JA, Friberg IM, Little S, Bradley JE. Review series on helminths, immune modulation and the hygiene hypothesis: immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies? Immunology. 2009;126:18–27. doi: 10.1111/j.1365-2567.2008.03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstock JV, Summers RW, Elliott DE, Qadir K, Urban JF, Jr., Thompson R. The possible link between de-worming and the emergence of immunological disease. J Lab Clin Med. 2002;139:334–8. doi: 10.1067/mlc.2002.124343. [DOI] [PubMed] [Google Scholar]
- 16.Torrero MN, Larson D, Hübner MP, Mitre E. CD200R surface expression as a marker of murine basophil activation. Clin Exp Allergy. 2009;39:361–9. doi: 10.1111/j.1365-2222.2008.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitre E, Taylor RT, Kubofcik J, Nutman TB. Parasite antigen-driven basophils are a major source of IL-4 in human filarial infections. J Immunol. 2004;172:2439–45. doi: 10.4049/jimmunol.172.4.2439. [DOI] [PubMed] [Google Scholar]
- 18.Torrero MN, Hübner MP, Larson D, Karasuyama H, Mitre E. Basophils Amplify Type 2 Immune Responses, but Do Not Serve a Protective Role, during Chronic Infection of Mice with the Filarial Nematode Litomosoides sigmodontis. J Immunol. 2010;185:7426–34. doi: 10.4049/jimmunol.0903864. [DOI] [PubMed] [Google Scholar]
- 19.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr., Dvorak AM, Finkelman FD, LeGros G, Paul WE. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–17. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2009;113:2816–25. doi: 10.1182/blood-2008-05-154773. [DOI] [PubMed] [Google Scholar]
- 21.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4(+) T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009 doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finnegan A, Mikecz K, Tao P, Glant TT. Proteoglycan (aggrecan)-induced arthritis in BALB/c mice is a Th1-type disease regulated by Th2 cytokines. J Immunol. 1999;163:5383–90. [PubMed] [Google Scholar]
- 23.Shaw MK, Lorens JB, Dhawan A, DalCanto R, Tse HY, Tran AB, Bonpane C, Eswaran SL, Brocke S, Sarvetnick N, Steinman L, Nolan GP, Fathman CG. Local delivery of interleukin 4 by retrovirus-transduced T lymphocytes ameliorates experimental autoimmune encephalomyelitis. J Exp Med. 1997;185:1711–4. doi: 10.1084/jem.185.9.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J Clin Invest. 2001;108:1865–73. doi: 10.1172/JCI13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–46. doi: 10.1016/j.jaci.2009.02.030. quiz 747-8. [DOI] [PubMed] [Google Scholar]
- 26.Uermosi C, Beerli RR, Bauer M, Manolova V, Dietmeier K, Buser RB, Kundig TM, Saudan P, Bachmann MF. Mechanisms of allergen-specific desensitization. J Allergy Clin Immunol. 2010;126:375–83. doi: 10.1016/j.jaci.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 27.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–8. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hewitt PM, Armstrong N, Bowrey P, Cherian M, Morris DL. Cimetidine prevents suppression of delayed hypersensitivity in an animal model of haemorrhagic shock. Injury. 2002;33:673–8. doi: 10.1016/s0020-1383(02)00095-5. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe N, Matsuda E, Masuda A, Nariai K, Shibasaki T. The effects of fexofenadine on eosinophilia and systemic anaphylaxis in mice infected with Trichinella spiralis. Int Immunopharmacol. 2004;4:367–75. doi: 10.1016/j.intimp.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Gibbs BF, Haas H, Wolff HH, Grabbe J. Early IgE-dependent release of IL-4 and IL-13 from leukocytes is restricted to basophils: a comparison with other granulocytes and mononuclear cells. Inflamm Res. 2000;49(Suppl 1):S9–10. doi: 10.1007/PL00000197. [DOI] [PubMed] [Google Scholar]
- 31.Devouassoux G, Foster B, Scott LM, Metcalfe DD, Prussin C. Frequency and characterization of antigen-specific IL-4- and IL-13- producing basophils and T cells in peripheral blood of healthy and asthmatic subjects. J Allergy Clin Immunol. 1999;104:811–9. doi: 10.1016/s0091-6749(99)70292-7. [DOI] [PubMed] [Google Scholar]
- 32.Aoki I, Kinzer C, Shirai A, Paul WE, Klinman DM. IgE receptor-positive non-B/non-T cells dominate the production of interleukin 4 and interleukin 6 in immunized mice. Proc Natl Acad Sci U S A. 1995;92:2534–8. doi: 10.1073/pnas.92.7.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poorafshar M, Helmby H, Troye-Blomberg M, Hellman L. MMCP-8, the first lineage-specific differentiation marker for mouse basophils. Elevated numbers of potent IL-4-producing and MMCP-8-positive cells in spleens of malaria-infected mice. Eur J Immunol. 2000;30:2660–8. doi: 10.1002/1521-4141(200009)30:9<2660::AID-IMMU2660>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 34.Cameron MJ, Arreaza GA, Zucker P, Chensue SW, Strieter RM, Chakrabarti S, Delovitch TL. IL-4 prevents insulitis and insulin-dependent diabetes mellitus in nonobese diabetic mice by potentiation of regulatory T helper-2 cell function. J Immunol. 1997;159:4686–92. [PubMed] [Google Scholar]
- 35.Rapoport MJ, Jaramillo A, Zipris D, Lazarus AH, Serreze DV, Leiter EH, Cyopick P, Danska JS, Delovitch TL. Interleukin 4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. J Exp Med. 1993;178:87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller R, Krahl T, Sarvetnick N. Pancreatic expression of interleukin-4 abrogates insulitis and autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 1996;184:1093–9. doi: 10.1084/jem.184.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Creusot RJ, Yaghoubi SS, Kodama K, Dang DN, Dang VH, Breckpot K, Thielemans K, Gambhir SS, Fathman CG. Tissue-targeted therapy of autoimmune diabetes using dendritic cells transduced to express IL-4 in NOD mice. Clin Immunol. 2008;127:176–87. doi: 10.1016/j.clim.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feili-Hariri M, Falkner DH, Gambotto A, Papworth GD, Watkins SC, Robbins PD, Morel PA. Dendritic cells transduced to express interleukin-4 prevent diabetes in nonobese diabetic mice with advanced insulitis. Hum Gene Ther. 2003;14:13–23. doi: 10.1089/10430340360464679. [DOI] [PubMed] [Google Scholar]
- 39.Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF, Jr., Weinstock JV. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G385–91. doi: 10.1152/ajpgi.00049.2002. [DOI] [PubMed] [Google Scholar]
- 40.Jenkins SJ, Allen JE. Similarity and diversity in macrophage activation by nematodes, trematodes, and cestodes. J Biomed Biotechnol. 2010;2010:262609. doi: 10.1155/2010/262609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaccone P, Phillips J, Conget I, Gomis R, Haskins K, Minty A, Bendtzen K, Cooke A, Nicoletti F. Interleukin-13 prevents autoimmune diabetes in NOD mice. Diabetes. 1999;48:1522–8. doi: 10.2337/diabetes.48.8.1522. [DOI] [PubMed] [Google Scholar]
- 42.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, Shapiro AM. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58:1302–11. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009 doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooke A. Th17 cells in inflammatory conditions. Rev Diabet Stud. 2006;3:72–5. doi: 10.1900/RDS.2006.3.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jain R, Tartar DM, Gregg RK, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, Zaghouani H. Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med. 2008;205:207–18. doi: 10.1084/jem.20071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torrero MN, Hubner MP, Larson D, Karasuyama H, Mitre E. Basophils amplify type 2 immune responses, but do not serve a protective role, during chronic infection of mice with the filarial nematode Litomosoides sigmodntis. J Immunol. doi: 10.4049/jimmunol.0903864. (in press) [DOI] [PubMed] [Google Scholar]
- 47.Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect Immun. 2007;75:397–407. doi: 10.1128/IAI.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaccone P, Fehervari Z, Jones FM, Sidobre S, Kronenberg M, Dunne DW, Cooke A. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol. 2003;33:1439–49. doi: 10.1002/eji.200323910. [DOI] [PubMed] [Google Scholar]
- 49.Lennon GP, Bettini M, Burton AR, Vincent E, Arnold PY, Santamaria P, Vignali DA. T cell islet accumulation in type 1 diabetes is a tightly regulated, cell-autonomous event. Immunity. 2009;31:643–53. doi: 10.1016/j.immuni.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]