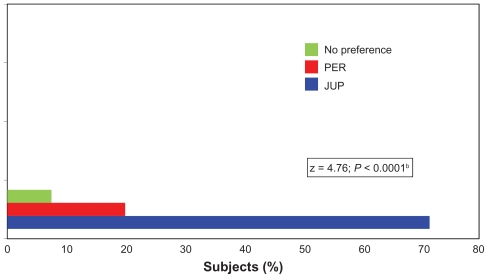
Figure 3.
Treatment preference, as evaluated by the subjectsa at the end of the study (Month 12) while still blinded to the treatment allocation.
Notes: a77/80 subjects who completed the 12-month study; bP value based on a difference in subject preference for Juvederm ULTRA PLUS™ (JUP) versus Perlane® (PER) using a one-sample test of proportions with a null hypothesis of an equal (50%) preference for missing data and a “no preference” response excluded from the analysis.
Abbreviations: JUP, Juvederm ULTRA PLUS™; PER, Perlane®.