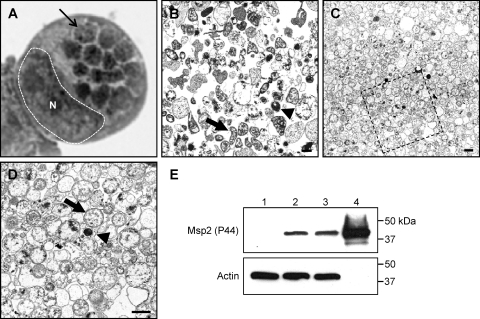
Fig. 1.
Dounce homogenization followed by discontinuous gradient centrifugation purifies A. phagocytophilum organisms while minimizing host cellular material contamination. (A) Light microscopic image of a Hema Fix-stained A. phagocytophilum-infected HL-60 cell that exhibits an infection load that is typical of the infected cells from which A. phagocytophilum organisms were purified for this study. A hatched line and an N demarcate the host cell nucleus, while a thin arrow denotes one of 14 morulae. (B) Transmission electron micrograph of A. phagocytophilum organisms and HL-60 cellular debris following syringe lysis and differential centrifugation to partially remove host cellular material. (C and D) Transmission electron micrograph of an A. phagocytophilum fraction obtained following centrifugation of a Dounce homogenate through a discontinuous Optiprep gradient. (D) Magnification of the region in panel C that is denoted by a hatched box. Thick arrows and arrowheads denote representative RC and DC organisms, respectively. Scale bars, 1 μm. (E) Western blot analyses demonstrating enrichment for A. phagocytophilum Msp2 (P44) by discontinuous gradient purification. Samples (10 μg) of whole-cell lysates of uninfected HL-60 cells (lane 1), A. phagocytophilum-infected HL-60 cells (lane 2), A. phagocytophilum organisms recovered following syringe lysis of infected HL-60 cells and differential centrifugation (lane 3), and A. phagocytophilum organisms recovered following Dounce homogenization and discontinuous density gradient centrifugation (lane 4) were resolved by SDS-PAGE, transferred to nitrocellulose, and screened with antibody against A. phagocytophilum Msp2 (P44) or human actin.