Abstract
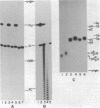
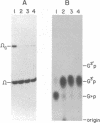
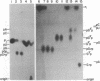
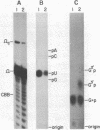
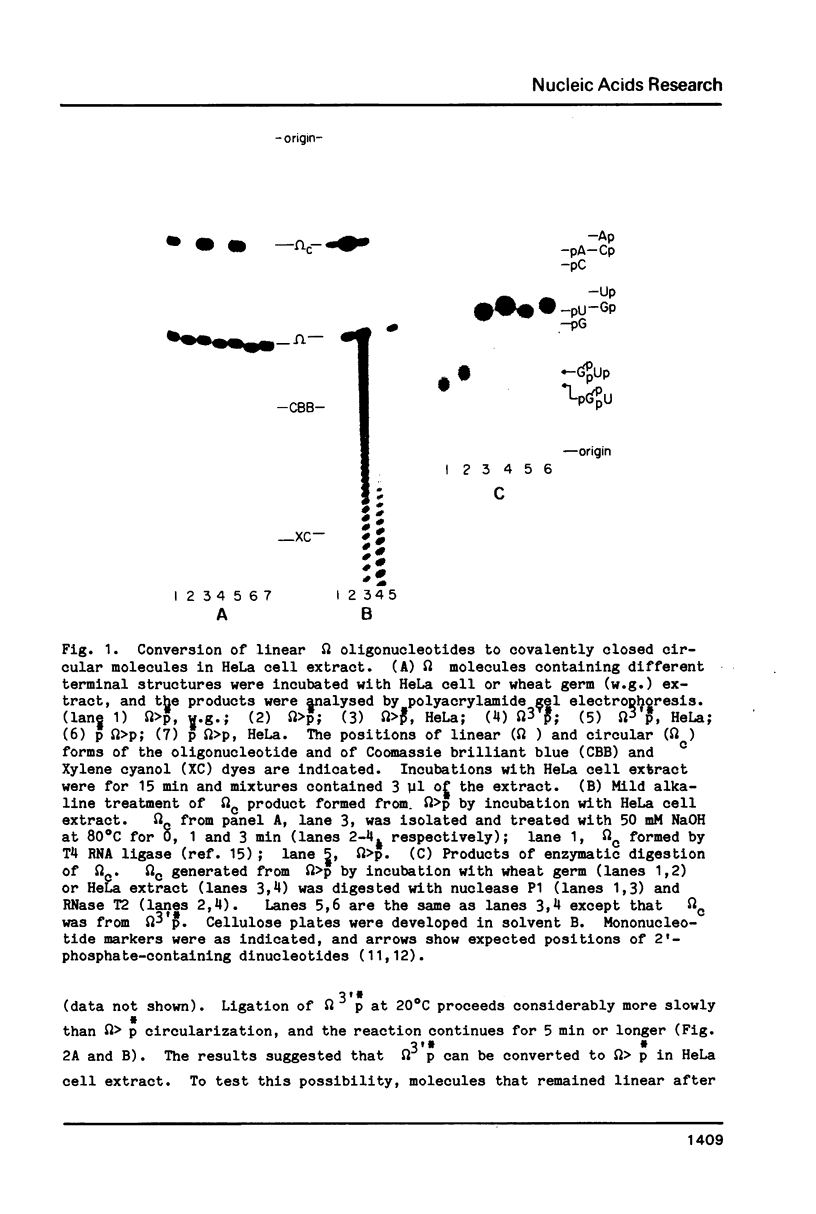
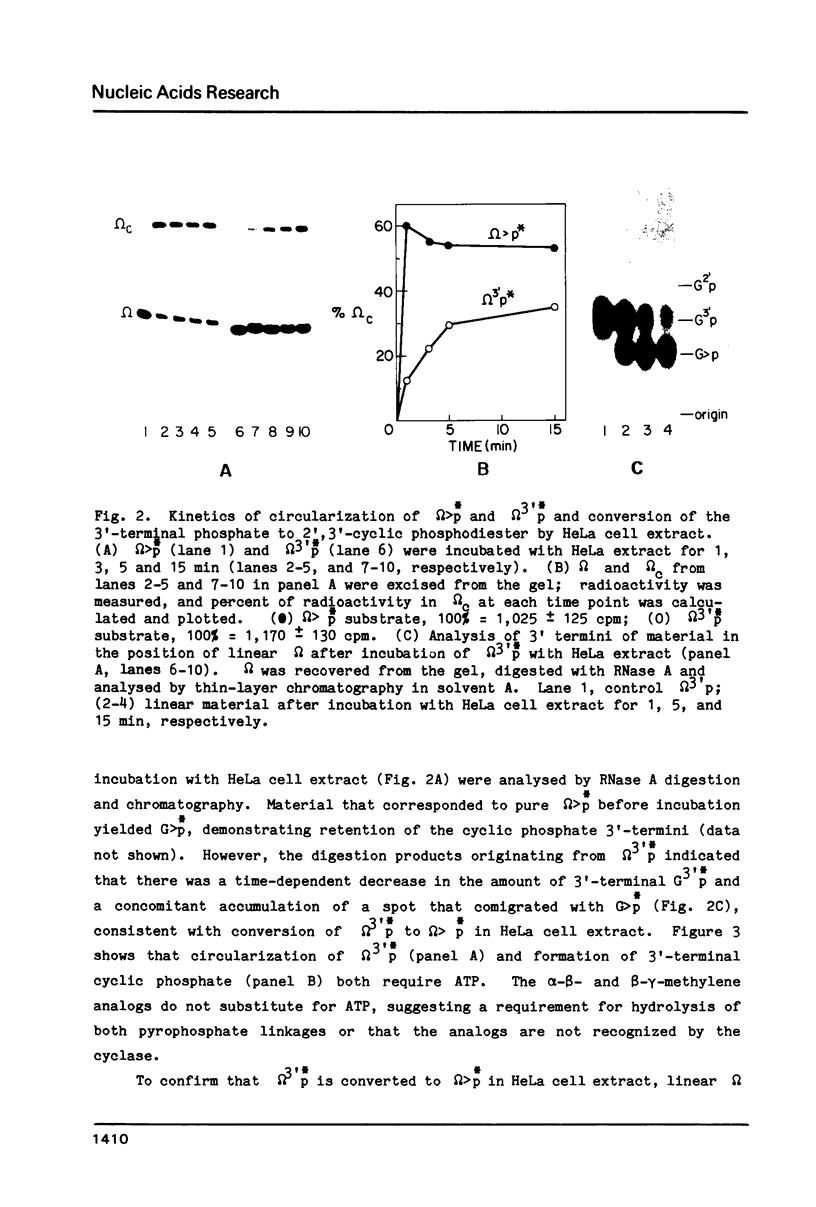
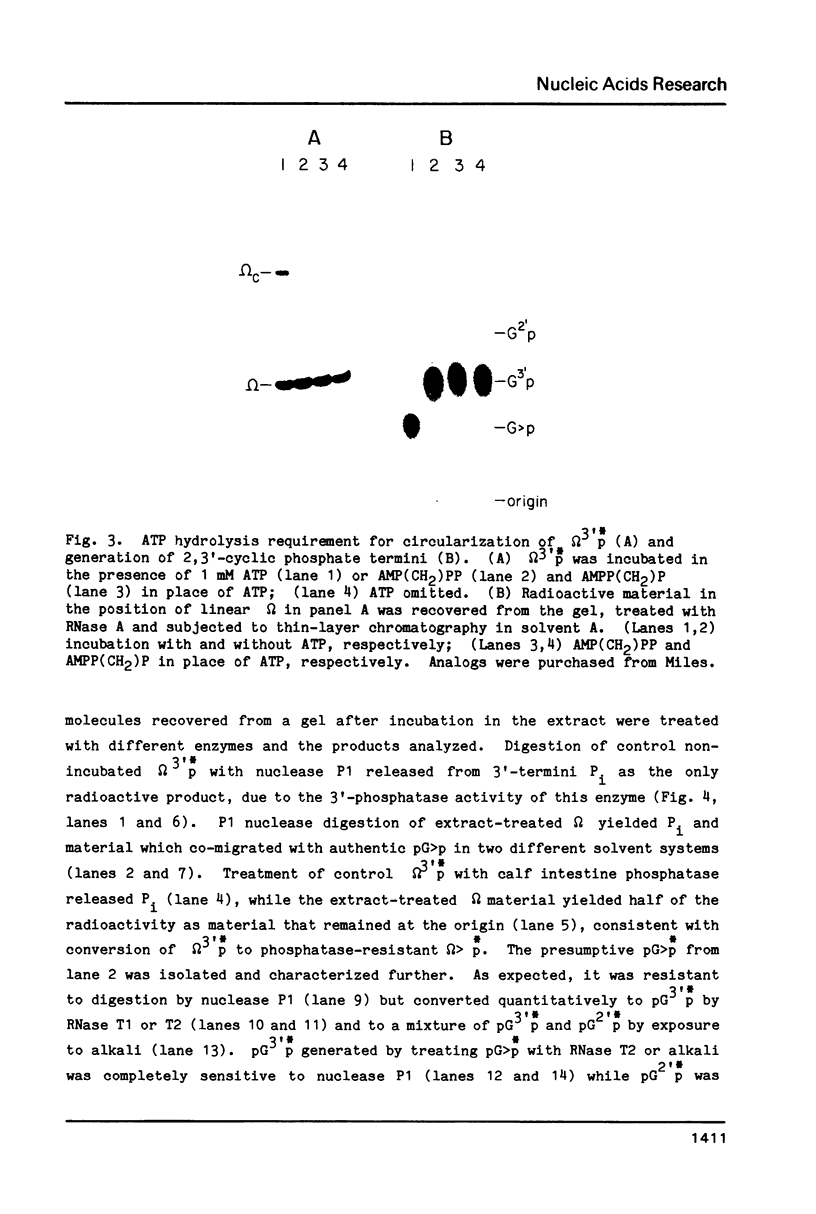
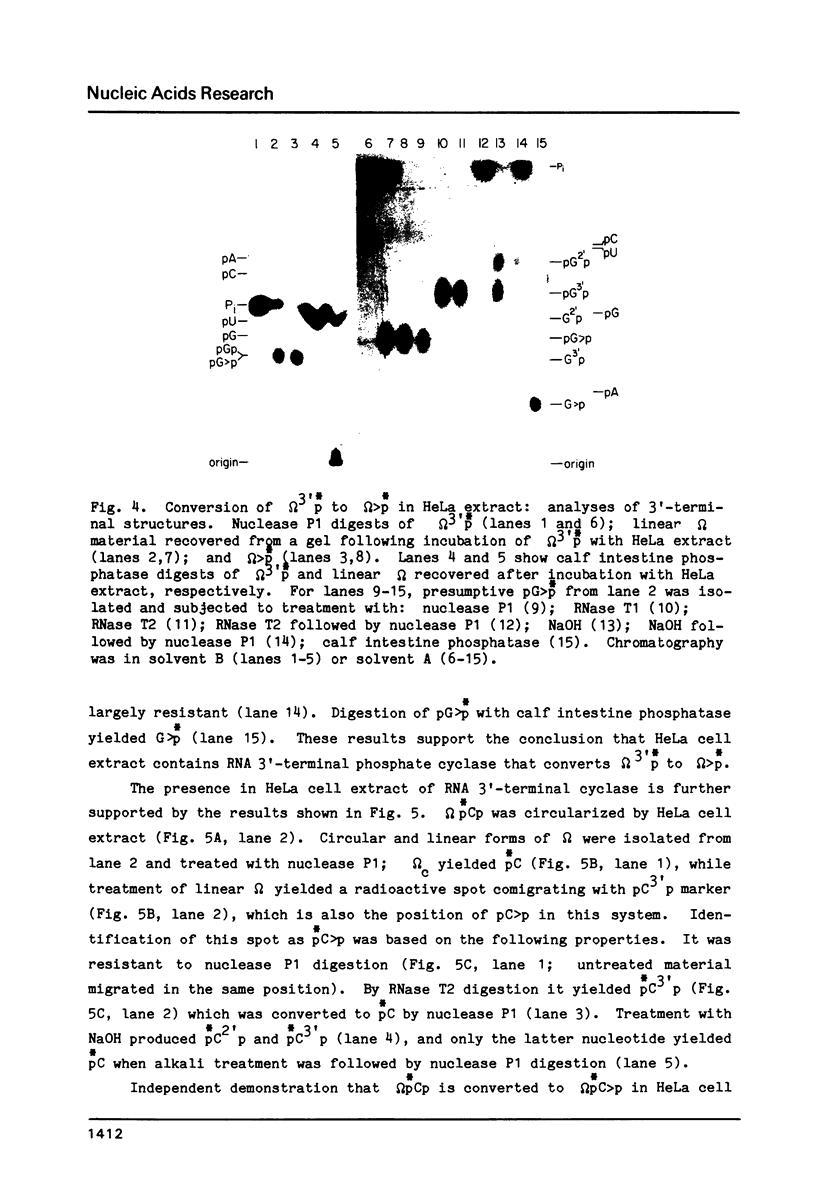
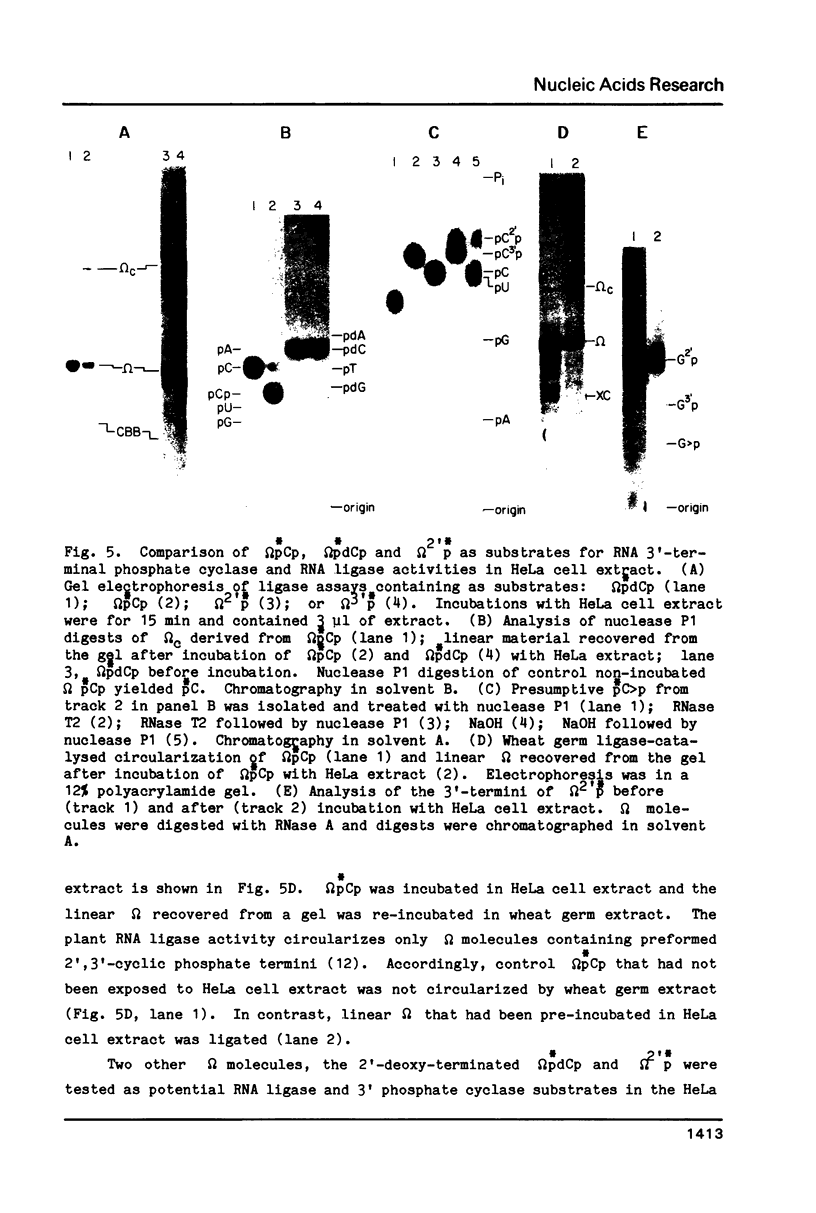
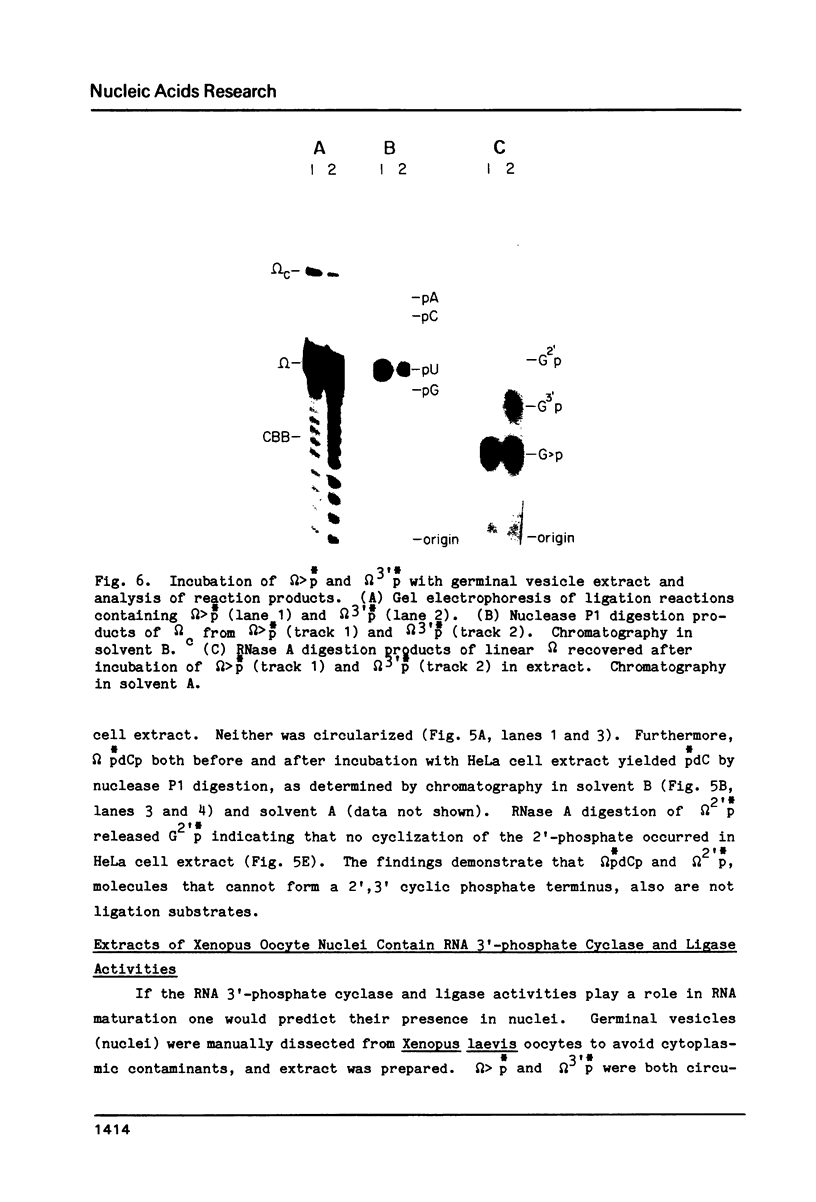
HeLa cell extract contains RNA ligase activity that converts linear polyribonucleotides to covalently closed circles. RNA substrates containing 2',3'-cyclic phosphate and 5'-hydroxyl termini are circularized by formation of a normal 3',5' phosphodiester bond. This activity differs from a previously described wheat germ RNA ligase which circularizes molecules with 2',3'-cyclic and 5' phosphate ends by a 2'-phosphomonester, 3',5'-phosphodiester linkage (Konarska et al., Nature 293, 112-116, 1981; Proc. Natl. Acad. Sci. USA 79, 1474-1478, 1982). The HeLa cell ligase can also utilize molecules with 3'-phosphate ends. However, in this case ligation is preceded by an ATP-dependent conversion of the 3'-terminal phosphate to the 2',3' cyclic form by a novel activity, RNA 3'-terminal phosphate cyclase. Both RNA ligase and RNA 3'-terminal phosphate cyclase activities are also present in extract of Xenopus oocyte nuclei, consistent with a role in RNA processing.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson J. RNA processing and the intervening sequence problem. Annu Rev Biochem. 1979;48:1035–1069. doi: 10.1146/annurev.bi.48.070179.005131. [DOI] [PubMed] [Google Scholar]
- Arnberg A. C., Van Ommen G. J., Grivell L. A., Van Bruggen E. F., Borst P. Some yeast mitochondrial RNAs are circular. Cell. 1980 Feb;19(2):313–319. doi: 10.1016/0092-8674(80)90505-x. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., Brown D. D., Jordan E. A nuclear extract of Xenopus laevis oocytes that accurately transcribes 5S RNA genes. Cell. 1978 Nov;15(3):1077–1086. doi: 10.1016/0092-8674(78)90291-x. [DOI] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D., Greer C., Gegenheimer P., Peebles C., Abelson J. Cell-free circularization of viroid progeny RNA by an RNA ligase from wheat germ. Science. 1982 Sep 17;217(4565):1147–1149. doi: 10.1126/science.217.4565.1147. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Zaug A. J., Grabowski P. J. In vitro splicing of the ribosomal RNA precursor of Tetrahymena: involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell. 1981 Dec;27(3 Pt 2):487–496. doi: 10.1016/0092-8674(81)90390-1. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M., Black P., Nishikura K. Intranuclear location of the tRNA splicing enzymes. Cell. 1981 Jan;23(1):89–93. doi: 10.1016/0092-8674(81)90273-7. [DOI] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. Enzymatic oligoribonucleotide synthesis with T4 RNA ligase. Biochemistry. 1978 May 30;17(11):2069–2076. doi: 10.1021/bi00604a008. [DOI] [PubMed] [Google Scholar]
- Filipowicz W., Shatkin A. J. Origin of splice junction phosphate in tRNAs processed by HeLa cell extract. Cell. 1983 Feb;32(2):547–557. doi: 10.1016/0092-8674(83)90474-9. [DOI] [PubMed] [Google Scholar]
- Grabowski P. J., Zaug A. J., Cech T. R. The intervening sequence of the ribosomal RNA precursor is converted to a circular RNA in isolated nuclei of Tetrahymena. Cell. 1981 Feb;23(2):467–476. doi: 10.1016/0092-8674(81)90142-2. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Halbreich A., Pajot P., Foucher M., Grandchamp C., Slonimski P. A pathway of cytochrome b mRNA processing in yeast mitochondria: specific splicing steps and an intron-derived circular DNA. Cell. 1980 Feb;19(2):321–329. doi: 10.1016/0092-8674(80)90506-1. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Comparative sequence and structure of viroid-like RNAs of two plant viruses. Nucleic Acids Res. 1982 Jun 25;10(12):3681–3691. doi: 10.1093/nar/10.12.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton D. M., Brennan C. A., Gumport R. I. The preparative synthesis of oligodeoxyribonucleotides using RNA ligase. Nucleic Acids Res. 1982 Mar 25;10(6):1877–1894. doi: 10.1093/nar/10.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann G., Littauer U. Z. Covalent joining of phenylalanine transfer ribonucleic acid half-molecules by T4 RNA ligase. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3741–3745. doi: 10.1073/pnas.71.9.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Tyc K., Filipowicz W., Sänger H. L., Gross H. J. Circularization of linear viroid RNA via 2'-phosphomonoester, 3', 5'-phosphodiester bonds by a novel type of RNA ligase from wheat germ and Chlamydomonas. Nucleic Acids Res. 1982 Dec 11;10(23):7521–7529. doi: 10.1093/nar/10.23.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M., Filipowicz W., Domdey H., Gross H. J. Binding of ribosomes to linear and circular forms of the 5'-terminal leader fragment of tobacco-mosaic-virus RNA. Eur J Biochem. 1981 Feb;114(2):221–227. doi: 10.1111/j.1432-1033.1981.tb05139.x. [DOI] [PubMed] [Google Scholar]
- Konarska M., Filipowicz W., Domdey H., Gross H. J. Formation of a 2'-phosphomonoester, 3',5'-phosphodiester linkage by a novel RNA ligase in wheat germ. Nature. 1981 Sep 10;293(5828):112–116. doi: 10.1038/293112a0. [DOI] [PubMed] [Google Scholar]
- Konarska M., Filipowicz W., Gross H. J. RNA ligation via 2'-phosphomonoester, 3'5'-phosphodiester linkage: requirement of 2',3'-cyclic phosphate termini and involvement of a 5'-hydroxyl polynucleotide kinase. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1474–1478. doi: 10.1073/pnas.79.5.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka A., de Paolis A., Tocchini-Valentini G. P. Ribonuclease "XlaI," an activity from Xenopus laevis oocytes that excises intervening sequences from yeast transfer ribonucleic acid precursors. Mol Cell Biol. 1981 Mar;1(3):269–280. doi: 10.1128/mcb.1.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles C. L., Ogden R. C., Knapp G., Abelson J. Splicing of yeast tRNA precursors: a two-stage reaction. Cell. 1979 Sep;18(1):27–35. doi: 10.1016/0092-8674(79)90350-7. [DOI] [PubMed] [Google Scholar]
- Sogin D. C. 2',3'-Cyclic NADP as a substrate for 2',3'-cyclic nucleotide 3'-phosphohydrolase. J Neurochem. 1976 Dec;27(6):1333–1337. doi: 10.1111/j.1471-4159.1976.tb02612.x. [DOI] [PubMed] [Google Scholar]
- Weil P. A., Segall J., Harris B., Ng S. Y., Roeder R. G. Faithful transcription of eukaryotic genes by RNA polymerase III in systems reconstituted with purified DNA templates. J Biol Chem. 1979 Jul 10;254(13):6163–6173. [PubMed] [Google Scholar]