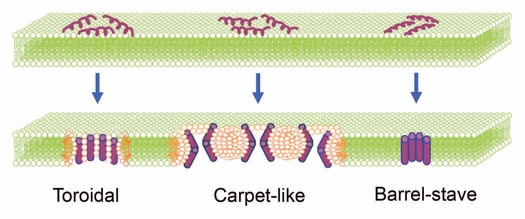
Figure 1.
Schematic illustration of three pore-forming mechanisms to explain the α-helical membrane peptides. In “toroidal” model, the peptide builds toroidal pores in lipid bilayers. Pore construction is managed by the lipid polar head groups and the helix bundles that orient vertically to the membrane exterior. In other words, the attached peptides aggregate and tempt the lipid monolayers to bend continuously through the pore so that both the inserted peptides and the lipid head groups line the water core. “Carpet-like” mechanism refers to destruction of membrane assembly by collaborative action of peptides. Peptides self-associate onto the acidic phospholipids-rich regions of lipid bilayers, and as soon as their concentrations reach to a certain threshold, they permeate into the membrane. This is assisted by escalating the positive potential of bilayer. Via “barrel-stave” mechanism, peptide inserts into the membrane hydrophobic substance, flips inward and creates a pore by forming transmembrane helical bundles. (Scheme is modified after refs. 1 and 46).