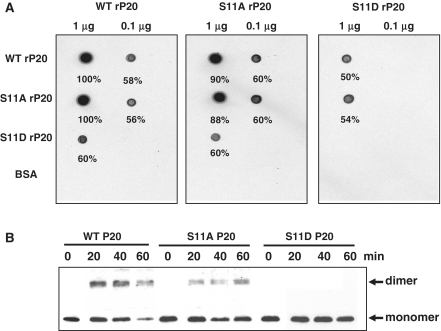
Figure 4.
Protein interactions of wild-type and mutant rP20. (A) Overlay assay. Purified WT, S11A or S11D rP20 was dot-blotted onto a PVDF membrane. After blocking with 1% BSA (w/v) in 140 mM NaCl, 10 mM Tris–HCl, pH 7.4, 2 mM EDTA, 0.1% Tween 20 (v/v) and 2 mM DTT at room temperature, the membrane was overlayed at 4°C with 35[S]-Met-labeled WT, S11A or S11D translated in vitro. After washing the membrane in Tris-buffered saline buffer containing 0.05% Tween 20 (v/v), protein interactions were detected by autoradiography. Bovine serum albumin was used as a control. (B) Glutaraldehyde cross-linking assay. Total protein extracted from N. benthamiana leaves co-infected with BaMV and WT, S11A or S11D satBaMV was cross-linked with 0.025% glutaraldehyde (v/v) for indicated times at 30°C, resolved on 12.5% SDS–PAGE and immunodetected with anti-P20 serum.