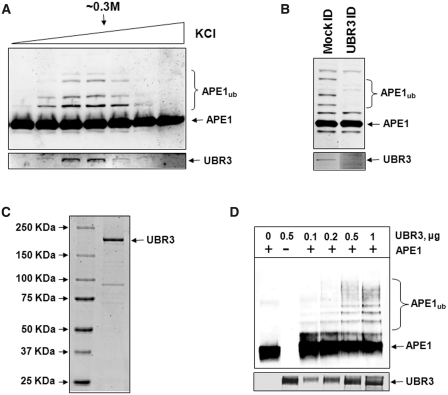
Figure 3.
UBR3 is the E3 ubiquitin ligase for APE1. (A) In vitro ubiquitylation of APE1 (2.8 pmol) by the final Mono-Q chromatography fractions purified from HeLa cells in the presence of E1 (0.7 pmol), UbcH2 (10.5 pmol) and ubiquitin (0.6 nmol) were analysed by 10% SDS–PAGE and immunoblotting using APE1 antibodies. Fractions containing APE1 ubiquitylation activity were shown to correlate with the presence of UBR3 protein detected by immunoblotting (lower panel). (B) The peak APE1 ubiquitylation activity containing fraction from the final Mono-Q chromatography was mock immunodepleted and immunodepleted with UBR3 antibodies. The fraction was subsequently analysed for in vitro ubiquitylation of APE1 (2.8 pmol) in the presence of E1 (0.7 pmol), UbcH2 (10.5 pmol) and ubiquitin (0.6 nmol) by 10% SDS–PAGE and immunoblotting using APE1 antibodies (upper panel). Furthermore, the pull-down was analysed by 10% SDS–PAGE and immunoblotting using UBR3 antibodies (lower panel). (C) N-terminal Flag-tagged mouse UBR3 was expressed in S. cerevisiae, purified and analysed by 4–12% SDS–PAGE and Coomassie staining. (D) Purified Flag-tagged mouse UBR3 was analysed for in vitro ubiquitylation of APE1 (2.8 pmol) in the presence of E1 (0.7 pmol), UbcH2 (10.5 pmol) and ubiquitin (0.6 nmol) by 10% SDS–PAGE and immunoblotting using APE1 antibodies and UBR3 antibodies (lower panel). Molecular weight markers are indicated on the left hand side of appropriate figures and the positions of ubiquitylated APE1 (APE1ub) are shown.