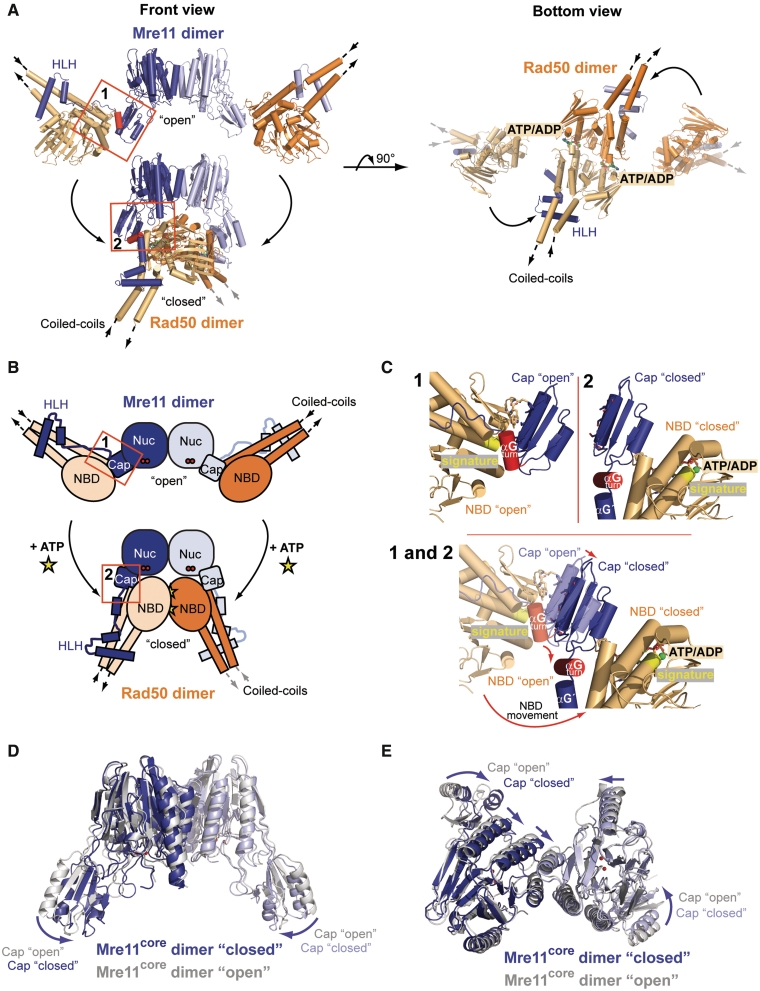
Figure 2.
Conformational change of the TmMRNBD complex upon nucleotide binding. (A) Comparison of the TmMRNBD complex structures in the open and ATP/ADP-bound conformation shown in two different orientations (colored according to Figure 1A). (B) Schematic representation of the overall domain movement within the complex upon ATP binding. Conformational changes in interface II are highlighted. (C) Close up view of the Mre11 capping domain and interface II in the open (1) and closed (2) TmMRNBD complex. The capping domains move inward by ∼4 Å due to their interaction with the Rad50 NBDs and helix αGturn rotates by roughly 90° from the open to the closed state. (D and E) Superposition of the TmMre11 core domains of the nucleotide bound (blue) and unbound (gray; PDB entry: 3QG5) structures. The arrows indicate the rotation of the capping domain toward the nuclease active site upon Rad50 ATP binding. (E) The bottom view highlights the slight changes in the arrangement of the four-helix bundle between the open and closed conformation (straight arrows).