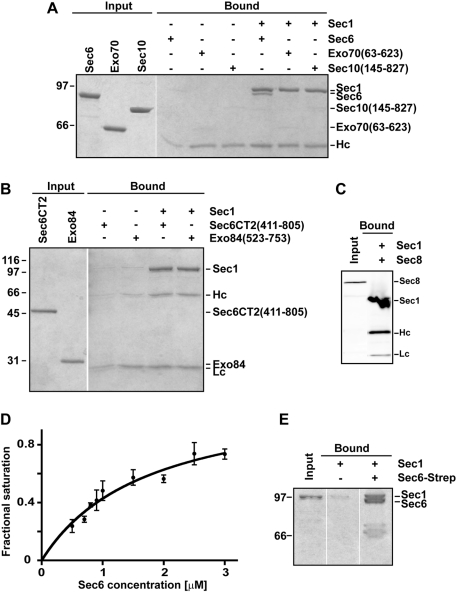
FIGURE 1:
Sec1 interacts with the exocyst subunit Sec6. (A) Purified Sec1-V5-His6 was immobilized with α-V5 antibody and mixed with purified exocyst subunits: Sec6, Exo70 (63–623), and Sec10 (145–827). Input and bound samples were run on an 8% SDS–PAGE gel and stained with Coomassie blue dye. (B) Similar experiments were performed using the C-terminal domain (411–805) of Sec6 and Exo84 (523–753). Input and bound samples were run on a 12% SDS–PAGE gel and stained with Coomassie blue dye. Only full-length Sec6 bound to Sec1. (C) Sec1-V5-His6 was immobilized and incubated with Ni NTA–purified His6-Sec8. In this case, the input and bound proteins (which are all His6 tagged) were analyzed by Western blotting using α-His5 antibody. (D) Representative binding curve for Sec6–Sec1. Sec1 was immobilized on beads and incubated with increasing concentrations of Sec6. The apparent binding constant was 1.7 ± 0.4 μM (mean ± SEM) from fits to three different experiments. (E) The Sec6–Sec1 binding interaction is reciprocal. Purified Strep-tagged Sec6 was immobilized on Strep-Tactin resin and incubated with purified Sec1. Input and bound samples were run on an 8% SDS–PAGE gel and stained with Coomassie blue dye. For all experiments, Hc and Lc refer to the heavy and light chains of the α-V5 antibody, respectively. To avoid leakage of proteins between different lanes, gels were often run with empty lanes between input and bound; empty lanes from the same gel were edited out using Photoshop. For all experiments, representative gels are shown for at least three repetitions.