In adults receiving buprenorphine-naloxone maintenance, buprenorphine pharmacokinetics were not changed significantly by 15-day coadministration of darunavir-ritonavir or fosamprenavir-ritonavir. The pharmacokinetics of the protease inhibitors did not differ from those in matched controls receiving only the protease inhibitors.
Abstract
Background. This study examined drug interactions between buprenorphine, a partial opioid agonist used for opioid dependence treatment and pain management, and the protease inhibitors (PIs) darunavir-ritonavir and fosamprenavir-ritonavir.
Methods. The pharmacokinetics of buprenorphine and its metabolites and symptoms of opioid withdrawal or excess were compared in opioid-dependent, buprenorphine-naloxone–maintained, human immunodeficiency virus (HIV)–negative volunteers (11 for darunavir-ritonavir and 10 for fosamprenavir-ritonavir) before and after 15 days of PI administration. PI pharmacokinetics and adverse effects were compared between the buprenorphine-maintained participants and an equal number of sex-, age-, race-, and weight-matched, healthy, non–opioid-dependent volunteers who received darunavir-ritonavir or fosamprenavir-ritonavir but not buprenorphine.
Results. There were no significant changes in buprenorphine or PI plasma levels and no significant changes in medication adverse effects or opioid withdrawal. Increased concentrations of the inactive metabolite buprenorphine-3-glucuronide suggested that darunavir-ritonavir and fosamprenavir-ritonavir induced glucuronidation of buprenorphine.
Conclusions. Dose adjustments are not likely to be necessary when buprenorphine and darunavir-ritonavir or fosamprenavir-ritonavir are coadministered for the treatment of opioid dependence and HIV disease.
Combination antiretroviral therapy (cART) is frequently underused in drug users with human immunodeficiency virus (HIV) disease because of difficulties in obtaining adherence adequate to maintain viral suppression [1]. Optimal clinical care requires treatment of both HIV disease and substance dependence. Opioid dependence can be effectively treated with either methadone or buprenorphine. However, several nonnucleoside reverse transcriptase inhibitors and protease inhibitors (PIs) have been shown to inhibit or induce methadone metabolism, resulting in potential for methadone toxicity or withdrawal symptoms [2].
Buprenorphine has been shown to be equivalent to methadone in the treatment of opioid-dependent patients [3] and can be prescribed by qualified physicians outside of specialized opioid dependence treatment programs. As a partial opioid agonist, buprenorphine has a ceiling effect that reduces toxicity at higher doses or when its metabolism is inhibited. Buprenorphine is metabolized in part via cytochrome P450 3A4 (CYP3A4) to norbuprenorphine [4, 5], an active metabolite, which may prevent opiate withdrawal when buprenorphine metabolism is induced. To date, the only clinically significant interaction found between buprenorphine and antiretrovirals is with atazanavir-ritonavir, which elevated buprenorphine and norbuprenophine concentrations [6].
Here we examine the interaction of buprenorphine with darunavir-ritonavir or fosamprenavir-ritonavir. When given alone, ritonavir, a potent inhibitor of CYP3A4 [7, 8], increases buprenorphine as well as norbuprenorphine concentrations [9] without increasing adverse effect of buprenorphine [10]. The effects of ritonavir in combination with other PIs are not necessarily predictable from the effects of the individual agents. For example, although darunavir is a mild CYP3A4 inhibitor and fosamprenavir a mixed inhibitor and inducer of CYP3A4 [11], darunavir-ritonavir decreases rather than increases methadone plasma levels, causing withdrawal symptoms [12], and fosamprenavir-ritonavir decreases R-methadone plasma levels, although without producing withdrawal symptoms [13]. These findings with methadone led us to ask how darunavir-ritonavir and fosamprenavir-ritonavir affect buprenorphine pharmacokinetics. We investigated (1) whether the pharmacokinetics of buprenorphine, administered as sublingual buprenorphine-naloxone, are affected by darunavir-ritonavir or fosamprenavir-ritonavir; (2) whether the pharmacokinetics of darunavir or fosamprenavir are affected by buprenorphine; and (3) whether clinically significant pharmacodynamic effects or toxic effects occur with coadministration.
METHODS
Design
Participants included (1) opioid-dependent adults (11 for darunavir-ritonavir, 10 for fosamprenavir-ritonavir) receiving a stable dose of buprenorphine-naloxone for ≥2 weeks and (2) equal numbers of sex-, age-, race-, and weight-matched, healthy, non–opioid-dependent volunteers. Both studies were open label and included (1) a within-subjects component that examined the effect of PI administration on buprenorphine disposition and (2) a between-subjects component that examined the effect of buprenorphine on PI disposition. The study design has been described elsewhere [14].
Procedures
The darunavir-ritonavir study was conducted at the University of California San Francisco (UCSF). The fosamprenavir-ritonavir study was conducted at UCSF and Virginia Commonwealth University. The studies were approved by the UCSF and both institutional review boards, respectively. Study fliers were posted in local substance abuse treatment clinics, in the community, and online. All participants provided voluntary written informed consent and received monetary compensation for their time. They received a physical and psychiatric evaluation. Substance use and mental disorders were diagnosed by means of clinical assessment and the Mini International Neuropsychiatric Interview [15]. Opioid-dependent participants received buprenorphine-naloxone and counseling at no charge. Those wishing to continue treatment at study exit were assisted with transfer to a community provider.
Eligible individuals were ≥18 years old, were not being treated with medications that might alter metabolic enzyme function, were HIV negative (by HIV antibody and viral load tests), and had no significant medical conditions as determined by medical history, physical examination, electrocardiogram, complete blood cell count, liver function tests (participants were excluded if their levels were ≥3 times the upper limit of normal), glucose test, urea nitrogen test, creatinine test, and pregnancy test (negative on entry and weekly during participation for women with childbearing potential). Urine was tested for recent use of opioids (including morphine, codeine, methadone, and oxycodone), cocaine, amphetamines, marijuana, and benzodiazepines. Urine toxicology was repeated before conducting pharmacokinetic studies and randomly, at least weekly, during study participation.
Opioid withdrawal was assessed by standardized clinician rating (Objective Opioid Withdrawal Scale [OOWS]; scores of ≥3 indicate moderate withdrawal symptoms) [16]). Cognitive impairment was measured with the Mini-Mental State Examination (MMSE; maximum score, 30; scores of <24 indicate cognitive impairment) [17]. Adverse experiences were recorded using an adverse symptoms checklist, including changes in energy, central nervous system effects, gastrointestinal symptoms, genitourinary symptoms, and other somatic complaints, each scored for severity on an ordinal scale (0–3; 0, not present; 1, mild; 2, moderate; 3, severe; maximum possible score; 87). These were administered before and at completion of PI administration.
Opioid-dependent participants were stabilized (defined as lack of opioid withdrawal, symptoms, or craving and cessation of opioid use as determined by urine toxicology) with a once-daily dose of sublingual buprenorphine-naloxone. After ≥2 weeks receiving this dose, they participated in a 24-hour study of buprenorphine pharmacokinetics. For the next 15 days they received buprenorphine-naloxone along with once-daily oral doses of darunavir-ritonavir (800 and 100 mg, respectively) or fosamprenavir-ritonavir (1400 and 200 mg, respectively). This was followed by another 24-hour study to determine buprenorphine and PI pharmacokinetics. For both the darunavir-ritonavir and fosamprenavir-ritonavir studies, an equal number of age-, weight-, race-, and sex-matched control participants received the same antiretroviral doses for 15 days followed by a 24-hour study of PI pharmacokinetics. Ingestion of all doses was observed by study staff.
Participants were admitted to the Clinical Research Center for pharmacokinetic studies. Results of breath alcohol tests and urine drug screens at admission had to be negative in order to proceed. Participants were fed a standardized breakfast 1 hour before the start of blood sampling. Blood samples were collected before buprenorphine-naloxone and PI administration and at 0.5, 1, 1.5, 2, 4, 6, 8, 12, and 24 hours after administration. Plasma samples were prepared soon after collection and stored frozen until time of analysis.
Biochemical Assays
Buprenorphine and metabolite concentrations were determined using liquid chromatography–tandem mass spectrometry as described elsewhere [18], except that buprenorphine-d4 and norbuprenorphine-d3 were used as the internal standards for their respective glucuronides. Darunavir [19] or amprenavir (the active metabolite of fosamprenavir) [20] were quantified using high-performance liquid chromatography assays, described elsewhere.
Pharmacokinetic Analysis
To facilitate summary and comparison, concentrations of buprenorphine and its metabolites were normalized to the concentrations expected for a 16-mg dose of buprenorphine by multiplying the measured concentration by 16 divided by the dose administered. Pharmacokinetic parameters for buprenorphine, norbuprenorphine, buprenorphine-3-glucuronide, norbuprenorphine-3-glucuronide, and either darunavir or amprenavir were determined as appropriate for each subject. The area under the concentration-time curve (AUC), trough plasma concentration (C24), maximum plasma concentration (Cmax), time of Cmax (Tmax), and bioavailability-adjusted clearance (CL/F) were determined using the noncompartmental analysis module of WinNonLin Professional software (version 3.2; Pharsight [21]). Drug concentrations that were less than the limit of quantitation were expressed as one-half of the limit for analysis. For metabolites, the value of CL/F was calculated with F as the fraction of parent drug dose converted to circulating metabolite.
Statistical Analysis
Past drug interaction studies of methadone and antiretroviral medications indicated that the coefficients of variation for PI parameters would be ∼30%. A sample size of 10 was needed to detect a 40% difference in PI AUC with a power of 0.8. Because within-subject coefficients of variation are smaller, a sample size of 7 was adequate to detect a 40% difference in buprenorphine AUC [22].
Buprenorphine pharmacokinetic parameters were compared within subjects by means of the paired t test, except for the nonparametric value Tmax, for which the Wilcoxon test was used. PI pharmacokinetic parameters for the buprenorphine group versus the control group were compared by means of the Kruskal–Wallis test, and those for Tmax were compared by means of the Mann–Whitney U test. Differences were considered statistically significant at P ≤.05 (2-tailed). Subject characteristics were compared by single-factor analysis of variance.
RESULTS
Participants
Most opioid-dependent participants were stabilized with 16 and 4 mg of buprenorphine and naloxone, respectively; 3 participants in the darunavir-ritonavir study were stabilized with lower doses (12:3, 10:2.5, and 8:2 mg, respectively), and 2 participants in the fosamprenavir-ritonavir study were stabilized with lower doses (8:2 and 4:1 mg, respectively). Injection drug use and hepatitis C were more frequent, and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels higher, in the participants who received darunavir-ritonavir than in those who received fosamprenavir-ritonavir (Table 1). Participants were otherwise physically healthy and had no mental disorders other than substance use disorders. Control subjects were matched by sex, race, age, and weight; were physically healthy; and had no mental disorders. Concomitant medication use was limited to 1 buprenorphine-naloxone–maintained participant in the darunavir-ritonavir study who was chronically treated with thyroid hormone supplement and had normal thyroid function.
Table 1.
Sample Characteristicsa
| Variable | Darunavir-Ritonavir and Buprenorphine Group (n = 11) | Darunavir-Ritonavir Control Group (n = 11) | Fosamprenavir-Ritonavir and Buprenorphine Group (n = 10) | Fosamprenavir-Ritonavir Control Group (n = 10) |
| Buprenorphine dose/naloxone dose, mg/d | 14.4 (0.9)/3.6 (0.2) | NA | 14.0 (1.4)/3.5 (0.3) | NA |
| Age, years | 46.3 (2.6) | 43.3 (3.0) | 40.3 (2.9) | 37.6 (2.8) |
| Weight, kg | 68.3 (3.4) | 78.8 (4.2) | 97.6 (9.5) | 80.5 (5.5) |
| Female sex, No. (%) of participants | 6 (54.5) | 3 (27.3) | 2 (20) | 3 (30) |
| Race, No. (%) of participants | ||||
| African American | 1 (9) | 5 (45.5) | 9 (90) | 6 (60) |
| White | 6 (54.5) | 6 (54.5) | 1 (10) | 4 (40) |
| Hispanic | 3 (27.3) | 0 (0) | … | … |
| Native American | 1 (9) | 0 (0) | … | … |
| Substance use disorders, No. (%) of participants | ||||
| Opioid dependence | 11 (100)b | 0 (0) | 10 (100)b | 0 (0) |
| Cocaine abuse | 2 (18) | 0 (0) | 2 (20) | 3 (30) |
| Alcohol abuse | 1 (9) | 0 (0) | 0 (0) | 1 (10) |
| Marijuana abuse | 1 (9) | 1 (9) | 0 (0) | 1 (10) |
| Amphetamine abuse | 1 (9) | 0 (0) | … | … |
| Injection drug use | 9 (81.8)b | 0 (0) | 2 (20) | 0 (0) |
| Nicotine use, packs per day | 0.7 (0.1)c | 0.2 (0.1) | 0.5 (0.1) | 0.23 (0.1) |
| Hepatitis C–positive, No. (%) of participants | 8 (72.7)c | 0 (0) | 2 (20) | 0 (0) |
| AST level, U/Ld | ||||
| Before PI therapy | 32.5 (6.5) | 29.9 (3.4) | 24.4 (2.5) | 26.8 (3.6) |
| After PI therapy | 30.6 (6.4) | 22.1 (1.0) | 22.7 (2.1) | 22.4 (2.1) |
| ALT level, U/Ld | ||||
| Before PI therapy | 31.9 (8.8) | 24.8 (4.6) | 23.5 (4.1) | 23.0 (2.7) |
| After PI therapy | 26.6 (7.6) | 17.6 (1.5) | 21.2 (3.4) | 20.0 (1.9) |
| ECG QTc interval, mse | ||||
| Before PI therapy | 417.9 (5.1) | 424.7 (4.8) | 407.4 (3.3) | 411.8 (4.5) |
| After PI therapy | 420.9 (4.6) | 422.8 (6.2) | 403.1 (3.3) | 409.9 (9.7) |
| ECG PR interval, msf | ||||
| Before PI therapy | 159.3 (8.0) | 157.3 (6.4) | 176.4 (7.3) | 159.0 (5.5) |
| After PI therapy | 161.8 (5.9) | 161.5 (7.1) | 180.0 (7.9) | 164.4 (6.2) |
| Adverse symptoms, No. (%) of participants | ||||
| Before PI therapy | 5.6 (2.1) | 1.5 (0.6) | 1.9 (0.8) | 0.6 (0.4) |
| After PI therapy | 6.1 (1.7) | 2.4 (1.0) | 2.7 (0.9)g | 0.5 (0.3) |
Data are mean (standard error) values, unless otherwise indicated. ALT, alanine aminotransferase; AST, aspartate aminotransferase; ECG, electrocardiogram; NA, not applicable; PI, protease inhibitor; QTc, corrected QT.
P = .0001.
P = .001.
Normal range, 0–35 U/L.
Normal range, ≤430 ms for men and ≤ 450 ms for women.
Normal range, 120–200 ms.
P = .04.
Abuse of substances other than opioids was common in both the buprenorphine and control groups, with cocaine abuse most prevalent (Table 1). No participants met criteria for dependence on drugs other than opioids. Moderate cigarette smoking was common in both opioid-dependent and control participants, with all smokers reporting 1 pack per day (PPD) or less (range, 0.1–1.0 PPD). In the darunavir-ritonavir study, the control group smoked significantly less than the opioid-dependent group (5 vs 10 smokers and fewer cigarettes per day among smokers).
Interactions Between Buprenorphine and PIs
Effects of Darunavir-Ritonavir on Buprenorphine
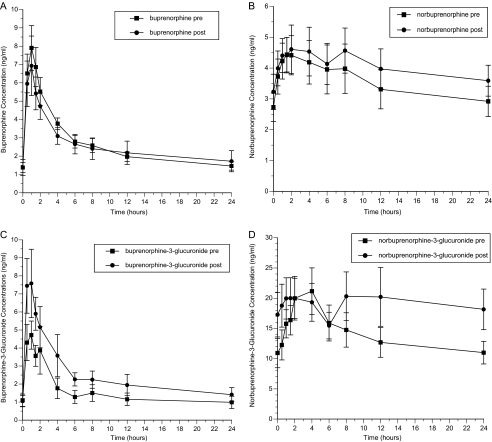
Darunavir-ritonavir produced no significant changes in the pharmacokinetics of buprenorphine or norbuprenorphine (Table 2; Figure 1). For buprenorphine-3-glucuronide, the values of AUC and Cmax increased and that of CL/F decreased significantly. The later Tmax for norbuprenorphine-3-glucuronide was clinically inconsequential. Darunavir-ritonavir administration did not increase opiate withdrawal (OOWS score both before and after darunavir-ritonavir, 0.0 [standard error, 0.0]) or cognitive problems (no MMSE scores of <24). Darunavir-ritonavir had no clinically significant effects on AST or ALT levels (Table 1), and corrected QT (QTc) and PR intervals at electrocardiography did not change significantly. Adverse symptoms were infrequent and did not differ significantly during administration of darunavir-ritonavir, compared with before administration, or between the participants who received buprenorphine-naloxone and the control subjects.
Table 2.
Effect of Darunavir-Ritonavir on Buprenorphine and Buprenorphine Metabolite Pharmacokineticsa
| Pharmacokinetic Parameter | Before Darunavir-Ritonavir Therapy (n = 11) | During Darunavir-Ritonavir Therapy (n = 11) | P Value |
| Buprenorphine | |||
| AUC0–24, h*ng/mL | 63.3 (6.3) | 61.9 (13.8) | .90 |
| CL/F, L/h | 279 (27) | 347 (49) | .11 |
| Cmax, ng/mL | 8.4 (1.2) | 7.3 (1.5) | .31 |
| Tmax, hours, median (range) | 1.0 (0.5–1.5) | 1.0 (0.5–1.5) | NS |
| C24, ng/mL | 1.46 (0.22) | 1.72 (0.58) | .58 |
| Norbuprenorphine | |||
| AUC0–24, h*ng/mL | 85 (14.4) | 98 (14.7) | .17 |
| CL/F, L/h | 239 (33) | 222 (49) | .67 |
| Cmax, ng/mL | 5.2 (0.8) | 5.4 (0.8) | .67 |
| Tmax, hours, median (range) | 1.5 (0.5–8) | 1.5 (0.5–8) | NS |
| C24, ng/mL | 2.9 (0.5) | 3.6 (0.5) | .09 |
| Buprenorphine-3-glucuronide | |||
| AUC0–24, h*ng/mL | 36 (9.0) | 61 (14.4) | .005 |
| CL/F, L/h | 744 (157) | 419 (88) | .005 |
| Cmax, ng/mL | 5.7 (0.8) | 9.7 (1.8) | .04 |
| Tmax, hours, median (range) | 0.5 (0.5–2) | 1.0 (0.5–4) | NS |
| C24, ng/mL | 0.99 (0.34) | 1.41 (0.39) | .12 |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24, h*ng/mL | 336 (55) | 466 (25) | .29 |
| CL/F, L/h | 57 (7) | 48 (8) | .41 |
| Cmax, ng/mL | 22.5 (3.7) | 24.3 (4.8) | .73 |
| Tmax, hours, median (range) | 4 (1.5–8) | 1.0 (0.5–4) | <.02 |
| C24, ng/mL | 11.0 (1.9) | 18.2 (3.4) | .14 |
Abbreviations: AUC, area under the concentration-time curve; C24, trough plasma concentration; Cmax, maximum plasma concentration; CL/F, bioavailability-adjusted clearance; NS, not significant; Tmax, time of Cmax.
Data are mean (standard error) values, unless otherwise indicated. All parameter values are adjusted to a standard dose of 16 mg of buprenorphine daily. The paired t test was used to determine P values for all parameters except Tmax, for which the Wilcoxon test was used.
Figure 1.
Effect of darunavir-ritonavir on plasma concentrations of buprenorphine (A), norbuprenorphine (B), buprenorphine-3-glucuronide (C), and norbuprenorphine-3-glucuronide (D).
Effects of Fosamprenavir-Ritonavir on Buprenorphine
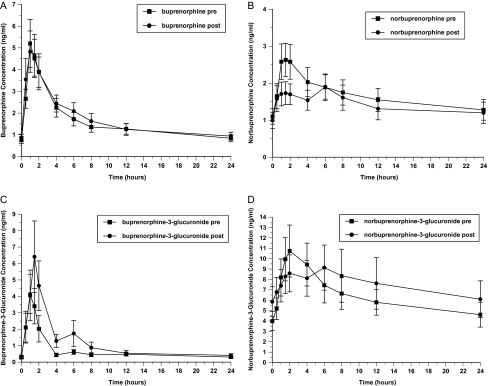
Fosamprenavir-ritonavir did not significantly affect the pharmacokinetics of buprenorphine or norbuprenorphine (Table 3; Figure 2). It did significantly increase the buprenorphine-3-glucuronide AUC. Fosamprenavir-ritonavir administration did not increase opioid withdrawal (OOWS score before fosamprenavir-ritonavir, 0.1; OOWS score after fosamprenavir-ritonavir, 0.0 [not significant]) or cognitive problems (no MMSE scores of <24). Fosamprenavir-ritonavir had no significant effects on AST level, ALT level, QTc interval, or PR interval (Table 1). Adverse symptoms were infrequent in both groups and did not change significantly during administration of fosamprenavir-ritonavir, compared with before administration.
Table 3.
Effect of Fosamprenavir-Ritonavir on Buprenorphine and Buprenorphine Metabolite Pharmacokineticsa
| Pharmacokinetic Parameter | Before Fosamprenavir-Ritonavir Therapy (n = 10) | During Fosamprenavir-Ritonavir Therapy (n = 10) | P Value |
| Buprenorphine | |||
| AUC0–24, h*ng/mL | 38.4 (7.0) | 40.9 (7.5) | .59 |
| CL/F, L/h | 628 (159) | 696 (219) | .60 |
| Cmax, ng/mL | 5.5 (1.1) | 5.3 (1.1) | .77 |
| Tmax, hours, median (range) | 1.0 (0.5–1.5) | 1.0 (0.5–1.5) | NS |
| C24, ng/mL | 0.84 (0.16) | 0.94 (0.18) | .35 |
| Norbuprenorphine | |||
| AUC0–24, h*ng/mL | 40.0 (7.8) | 33.9 (6.9) | .27 |
| CL/F, L/h | 751 (251) | 1063 (408) | .14 |
| Cmax, ng/mL | 2.9 (0.5) | 2.1 (0.4) | .67 |
| Tmax, hours, median (range) | 1.75 (1.0–2.0) | 2.0 (0.5–12) | NS |
| C24, ng/mL | 1.3 (0.3) | 1.2 (0.3) | .82 |
| Buprenorphine-3-glucuronide | |||
| AUC0–24, h*ng/mL | 16.4 (3.8) | 26.8 (6.3) | .03 |
| CL/F, L/h | 1513 (308) | 1300 (387) | .22 |
| Cmax, ng/mL | 4.9 (1.6) | 7.1 (2.1) | .26 |
| Tmax, hours, median (range) | 1.25 (0.5–12) | 1.5 (1.0–6) | NS |
| C24, ng/mL | 0.29 (0.06) | 0.37 (0.10) | .42 |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24, h*ng/mL | 154 (34) | 179 (49) | .51 |
| CL/F, L/h | 191 (65) | 314 (164) | .25 |
| Cmax, ng/mL | 11.3 (2.4) | 11.1 (2.5) | .92 |
| Tmax, hours, median (range) | 2.0 (1.0–12) | 2.0 (0.0–12) | NS |
| C24, ng/mL | 4.6 (1.2) | 6.1 (1.8) | .32 |
Abbreviations: AUC, area under the concentrationtime curve; C24, trough plasma concentration; Cmax, maximum plasma concentration; CL/F, bioavailability-adjusted clearance; NS, not significant; Tmax, time of Cmax.
Data are mean (standard error) values, unless otherwise indicated. All parameter values are adjusted to a standard dose of 16 mg of buprenorphine daily.
Figure 2.
Effect of fosamprenavir-ritonavir on plasma concentrations of buprenorphine (A), norbuprenorphine (B), buprenorphine-3-glucuronide (C), and norbuprenorphine-3-glucuronide (D).
Effects of Buprenorphine on PI Pharmacokinetics
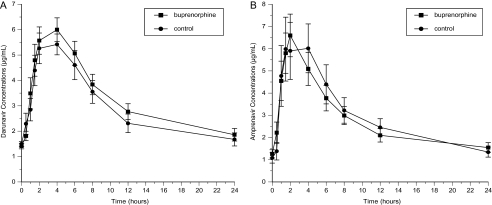
Buprenorphine-naloxone had no significant effects on the disposition of darunavir or amprenavir (Figure 3; Table 4). Concentrations of both PIs remained within their respective therapeutic ranges during buprenorphine-naloxone treatment.
Figure 3.
Effect of buprenorphine on plasma concentrations of darunavir (A) and amprenavir (B).
Table 4.
Effect of Buprenorphine on Darunavir and Amprenavir Concentrationsa
| Pharmacokinetic Parameter | Buprenorphine-Naloxone Group | Control Group | P Value |
| Darunavir (n = 11 in each group) | |||
| AUC0–24, h*μg/mL | 79.4 (18.0) | 71.0 (19.7) | .31 |
| CL/F, L/h | 10.54 (2.32) | 11.94 (2.74) | .21 |
| Cmax, μg/mL | 7.2 (1.2) | 6.9 (1.4) | .54 |
| Tmax, hours, median (range) | 4.0 (1.0–6.0) | 2.0 (1.5–6.0) | .85 |
| T1/2, hours | 18.7 (8.1) | 15.9 (9.8) | .28 |
| Amprenavir (active metabolite of fosamprenavir; n = 10 in each group) | |||
| AUC0–24, h*μg/mL | 67.4 (24.3) | 71.2 (36.3) | .79 |
| CL/F, L/h | 24.5 (12.6) | 29.2 (24.0) | .59 |
| Cmax, μg/mL | 7.2 (2.7) | 7.5 (4.7) | .86 |
| Tmax, hours, median (range) | 2.5 (1.0–4.0) | 3.5 (1.0–4.0) | .34 |
| T1/2, hours | 23.1 (15.0) | 17.3 (9.7) | .32 |
Abbreviations: AUC, area under the concentration time curve; C24, trough plasma concentration; Cmax, maximum plasma concentration; CL/F, bioavailability-adjusted clearance; Tmax, time of Cmax; T1/2, elimination half-life.
Data are mean (standard error) values, unless otherwise indicated. All parameter values are adjusted to a standard dose of 16 mg of buprenorphine daily.
DISCUSSION
Summary of Findings and Replication
The AUCs of buprenorphine and norbuprenorphine and the peak and trough concentrations did not change significantly with either PI combination, and no pharmacodynamic interactions were observed. The only significant change with these PIs was increased AUC of an inactive metabolite, buprenorphine-3-glucuronide. Darunavir and amprenavir pharmacokinetics were unaffected by buprenorphine. The AUC of buprenorphine also did not increase significantly in a similar study of 7 days of darunavir-ritonavir (600 and 100 mg, respectively, twice daily), a high dose typically given to therapy-experienced patients [12].
Glucuronidation
Ritonavir, darunavir, and fosamprenavir are all able to inhibit CYP3A4 [11], but we observed no significant effect of darunavir-ritonavir or fosamprenavir-ritonavir on buprenorphine AUC, which suggests possible induction of alternate clearance pathways. For example, the increases seen in the levels of buprenorphine glucuronide metabolites are consistent with induction of glucuronidation. The buprenorphine-3-glucuronide AUC increased with either darunavir-ritonavir or fosamprenavir-ritonavir in the present study, but not with ritonavir alone in our previous study [10]. Similarly, when darunavir-ritonavir was administered with etravirine, also a CYP3A4 substrate, the etravirine AUC decreased by 37%, suggesting induction of other drug-metabolizing enzymes [23]. The same pattern of unchanged buprenorphine level and increased buprenorphine-3-glucuronide level was also found for another boosted PI, lopinavir-ritonavir [10]. Induction of glucuronidation could become a problem if it decreases concentrations and clinical effectiveness of numerous other medications metabolized by this pathway, such as morphine, naloxone, oxazepam, zidovudine [24], nonsteroidal anti-inflammatory drugs, and antineoplastic agents [25].
Contrast to Interactions With Methadone
Our finding that darunavir-ritonavir and fosamprenavir-ritonavir did not significantly affect buprenorphine concentrations contributes to the advantage of buprenorphine-naloxone over methadone for the treatment of opioid dependence in HIV-positive patients. Darunavir-ritonavir and fosamprenavir-ritonavir decreased the μ-receptor active R-methadone AUC (16% and 18%, respectively), resulting in opiate withdrawal symptoms in the darunavir-ritonavir group (25%) [12] but no withdrawal in the fosamprenavir study [13]. Whereas some patients will need methadone dose increases when treated with darunavir-ritonavir or fosamprenavir-ritonavir, buprenorphine-naloxone doses will rarely require adjustment. If a methadone dose is increased during therapy with darunavir-ritonavir or fosamprenavir-ritonavir, then stopping therapy will create a risk for methadone toxicity and require tapering methadone back to a lower dose. This is unlikely to be necessary with buprenorphine-naloxone treatment.
Limitations
We studied the PIs without the other medications typically used in cART regimens. Studies of particular multidrug combinations could become obsolete before publication as the field of HIV treatment advances. We chose to study single-drug interactions because this information can help clinicians determine which medications might be responsible when adverse events occur.
Participants had opioid dependence but not HIV infection and/or AIDS. It would be difficult to enroll multiple individuals with HIV disease who all receive the same cART regimen, as well as buprenorphine-naloxone, who would (1) be healthy enough to safely participate and (2) not be receiving potentially interacting concomitant medications, making it difficult to complete a study in a timely manner. We have shown in previous studies that pharmacokinetics for zidovudine [26] and nevirapine [27] are similar in those with and without HIV infection.
Participants receiving buprenorphine and control participants were imperfectly matched. However, we found no significant differences in PI pharmacokinetics between buprenorphine-naloxone and control participants. It is unlikely that modest differences in the composition of the samples would have by chance offset a real effect of buprenorphine on the disposition of the PIs. In analyses of the effects of the PIs on buprenorphine pharmacokinetics and pharmacodynamics, subjects served as their own controls.
The small sample size was sufficient to detect pharmacokinetic differences, but it may have been too small to identify pharmacodynamic differences, which were infrequent and measured with categorical variables, reducing statistical power. However, major differences are unlikely, given that there were no significant pharmacodynamic trends, and the only significant pharmacokinetic change was the increase in levels of inactive buprenorphine-3-glucuronide.
CONCLUSIONS
This study demonstrated that there are no clinically significant pharmacokinetic or pharmacodynamic interactions between buprenorphine-naloxone and darunavir-ritonavir or fosamprenavir-ritonavir. The standard doses of darunavir-ritonavir and fosamprenavir-ritonavir used in clinical care of HIV infection may be given to opioid-dependent patients maintained with buprenorphine-naloxone without significant drug interactions. In contrast to methadone, buprenorphine-naloxone is unlikely to need a dose adjustment when patients start or stop taking darunavir-ritonavir or fosamprenavir-ritonavir. In addition, these PIs will continue to be effective when patients start taking buprenorphine-naloxone. Studies to date have shown no clinically significant interactions between buprenorphine and antiretroviral medications, except for atazanavir [6]. Thus, the present study contributes to the literature showing that, compared with methadone, buprenorphine simplifies and improves the safety profile for treatment for opioid dependence in those with HIV infection and/or AIDS.
Notes
Acknowledgments.
We appreciate the efforts of the staff at the Opiate Treatment Outpatient Program and the Integrated Buprenorphine Intervention Service of the San Francisco Department of Public Health, who referred potential participants to the study and accepted participants from the study who wished to continue buprenorphine treatment.
Financial support.
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (grants R01 DA 13004 and K24 DA 23359 to E.M.K.; grant R01 DA 10100 to D.E.M.; grant NCRR/NIH M01RR00065 for the General Clinical Research Center at Virginia Commonwealth University; and grant NIH/NCRR UCSF-CTSI UL1 RR024131 for the General Clinical Research Center at the University of California San Francisco).
Potential conflicts of interest.
D. E. M. has received research funding from Reckitt Benckiser, the manufacturer of buprenorphine-naloxone, for studies unrelated to the present study. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV infections. J Acquir Immune Defic Syndr. 2001;27:251–9. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- 2.McCance-Katz EF, Sullivan LS, Nallani S. Drug interactions of clinical importance between the opioids, methadone and buprenorphine, and frequently prescribed medications: a review. Am J Addict. 2010;19:4–16. doi: 10.1111/j.1521-0391.2009.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Buprenorphine versus methadone in the treatment of opioid dependence: self-reports, urinalysis, and addiction severity index. J Clin Psychopharmacol. 1996;16:58–67. doi: 10.1097/00004714-199602000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Iribarne C, Picart D, Dreano Y, Bail JP, Berthou F. Involvement of cytochrome P450 3A4 in N-dealkylation of buprenorphine in human liver microsomes. Life Sci. 1997;60:1953–64. doi: 10.1016/s0024-3205(97)00160-4. [DOI] [PubMed] [Google Scholar]
- 5.Moody DE, Slawson MH, Strain EC, Laycock JD, Spanbauer AC, Foltz RL. A liquid chromatographic-electrometric method for determination of buprenorphine, its metabolite, norbuprenorphine, and a conformulant, naloxone, that is suitable for in vivo and in vitro metabolism studies. Anal Biochem. 2002;306:31–9. doi: 10.1006/abio.2002.5673. [DOI] [PubMed] [Google Scholar]
- 6.McCance-Katz EF, Moody DE, Morse GD, et al. Interaction between buprenorphine and atazanavir or atazanavir/ritonavir. Drug Alcohol Depend. 2007;91:269–278. doi: 10.1016/j.drugalcdep.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu A, Granneman GR, Bertz RJ. Ritonavir: clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet. 1998;35:275–91. doi: 10.2165/00003088-199835040-00002. [DOI] [PubMed] [Google Scholar]
- 8.Kumar GN, Dykstra J, Roberts EM, et al. Potent inhibition of the cytochrome P-450 3A-mediated human liver microsomal metabolism of a novel HIV protease inhibitor by ritonavir: a positive drug-drug interaction. Drug Metab Dispos. 1999;27:902–8. [PubMed] [Google Scholar]
- 9.Iribarne C, Berthou F, Carlhant D, et al. Inhibition of methadone and buprenorphine N-dealkylations by three HIV-1 protease inhibitors. Drug Metab Dispos. 1998;26:257–60. [PubMed] [Google Scholar]
- 10.McCance-Katz EF, Moody DE, Smith PF, et al. Interactions between buprenorphine and antiretrovirals. II. The protease inhibitors nelfinavir, lopinavir/ritonavir, and ritonavir. Clin Infect Dis. 2006;43(Suppl 4):S235–46. doi: 10.1086/508188. [DOI] [PubMed] [Google Scholar]
- 11. Department of Health and Human Services, Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. www.aidsinfo.nih.gov/contentfiles/adultandadolescentGL.pdf. Accessed 30 October 2011.
- 12.Sekar V, Tomaka F, Lefebvre E, et al. Pharmacokinetic interactions between darunavir/ritonavir and opioid maintenance therapy using methadone or buprenorphine/naloxone. J Clin Pharmacol. 2011;51:271–8. doi: 10.1177/0091270010365558. [DOI] [PubMed] [Google Scholar]
- 13.Cao YJ, Smith PF, Wire MB, et al. Pharmacokinetics and pharmacodynamics of methadone enantiomers after coadministration with fosamprenavir-ritonavir in opioid-dependent subjects. Pharmacotherapy. 2008;28:863–74. doi: 10.1592/phco.28.7.863. [DOI] [PubMed] [Google Scholar]
- 14.McCance-Katz EF, Rainey PM, Friedland G, Jatlow P. The protease inhibitor lopinavir/ritonavir may produce opiate withdrawal in methadone-maintained patients. Clin Infect Dis. 2003;37:476–82. doi: 10.1086/376907. [DOI] [PubMed] [Google Scholar]
- 15.Sheehan DV, Lecrubier Y, Sheehan HK, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 16.Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Huang W, Moody DE, McCance-Katz EF. The in vivo glucuronidation of buprenorphine and norbuprenorphine determined by liquid chromatography-electrospray ionization-tandem mass spectrometry. Ther Drug Monit. 2006;28:245–51. doi: 10.1097/01.ftd.0000197094.92559.b4. [DOI] [PubMed] [Google Scholar]
- 19.Keil K, Hochreiter J, DiFrancesco R, et al. Integration of atazanavir into an existing liquid chromatography UV method for protease inhibitors: validation and application. Ther Drug Monit. 2007;29:103–9. doi: 10.1097/FTD.0b013e3180318ef3. [DOI] [PubMed] [Google Scholar]
- 20.Keil K, Frerichs VA, DiFrancesco R, Morse GD. Reverse phase high performance liquid chromatography method for the analysis of amprenavir, efavirenz, indinavir, lopinavir, nelfinavir and its active metabolite, M8, ritonavir, and saquinavir in heparinized human plasma. Ther Drug Monit. 2003;25:340–6. doi: 10.1097/00007691-200306000-00015. [DOI] [PubMed] [Google Scholar]
- 21.WinNonlin Professional Software, Version 3.2. Sunnyvale, CA: Pharsight Corporation; 1995. [Google Scholar]
- 22.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Hove and London; 1988. [Google Scholar]
- 23.Scholler-Gyure M, Kakuda TN, Sekar V, et al. Pharmacokinetics of darunavir/ritonavir and TMC 125 alone and co-administered in HIV negative volunteers. Antivir Ther. 2007;12:789–96. [PubMed] [Google Scholar]
- 24.Williams JA, Hyland R, Jones BC, et al. Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos. 2004;32:1201–8. doi: 10.1124/dmd.104.000794. [DOI] [PubMed] [Google Scholar]
- 25.Miners JO, Mackenzie PI, Knights KM. The prediction of drug-glucuronidation parameters in humans: UDP-glucuronosyltransferase enzyme-selective substrate and inhibitor probes for reaction phenotyping and in vitro-invivo extrapolation of drug clearance and drug-drug interaction potential. Drug Metab Rev. 2010;42:196–208. doi: 10.3109/03602530903210716. [DOI] [PubMed] [Google Scholar]
- 26.McCance-Katz EF, Rainey PM, Friedland G, Kosten TR, Jatlow P. Effect of opioid dependence pharmacotherapies on zidovudine disposition. Am J Addict. 2001;10:296–307. [PubMed] [Google Scholar]
- 27.McCance-Katz EF, Moody DE, Morse GD, Ma Q, Rainey PM. Lack of clinically significant drug interactions between nevirapine and buprenorphine. Am J Addict. 2010;19:30–7. doi: 10.1111/j.1521-0391.2009.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]