Abstract
PURPOSE OF REVIEW
Diarrheal disease causes substantial morbidity and mortality worldwide, however defining the microbiologic etiology is challenging due to the large number of potential enteropathogens that require testing, insensitivity of existing conventional methods, the frequent occurrence of mixed infections, and high rates of background carriage in many communities.
RECENT FINDINGS
Here we review recent detection methods for enteropathogens with a particular focus on nucleic acid amplification assays.
SUMMARY
Nucleic acid amplification assays with high sensitivity and throughput now allow screening for multiple enteropathogens in stool samples. Interpretation will be complicated by high rates of mixed infections and background carriage in many communities. Therefore new detection techniques, including quantitative methods, will need to be utilized in conjunction with the clinical context and careful study design. These methods should yield new insights into the etiology and epidemiology of diarrhea.
Keywords: Diarrhea, enteropathogen, nucleic acid amplification, PCR, real-time PCR
Introduction
Diarrheal disease causes a substantial burden of morbidity and mortality worldwide. It is estimated that diarrhea kills 1.5 million children each year, or 15% of attributable deaths [1,2]. Beyond mortality, morbidity from diarrheal diseases exacts an enormous global public health burden through growth faltering, malnutrition, and cognitive impairment [3]. Developed countries also experience a substantial health care burden due to diarrheal disease. In the United States alone an estimated $6 billion is lost annually for medical expenses and diminished productivity [4]. For all of these reasons, ascertaining the etiology of diarrhea is important in order to guide global health interventions, direct public health efforts in cases of outbreak situations, and care for patients.
Unfortunately there is no gold standard for the etiologic cause of diarrhea. The list of enteropathogens that can cause diarrhea is long and includes viruses, bacteria, protozoa, and helminths [4–6]. Even in the best-equipped clinical laboratories not every enteropathogen can be routinely tested. Furthermore, detection does not denote disease, since virtually all enteropathogens can exist asymptomatically or subclinically, particularly in developing country settings. Thus the interpretation of a detected enteropathogen in a stool sample of an individual with diarrhea must be made cautiously, since our detection schemes can never be completely comprehensive and detected pathogens may not be the causative ones. In this context, this review will describe evolving strategies to discern the etiology of diarrhea.
The Clinical-Laboratory Context
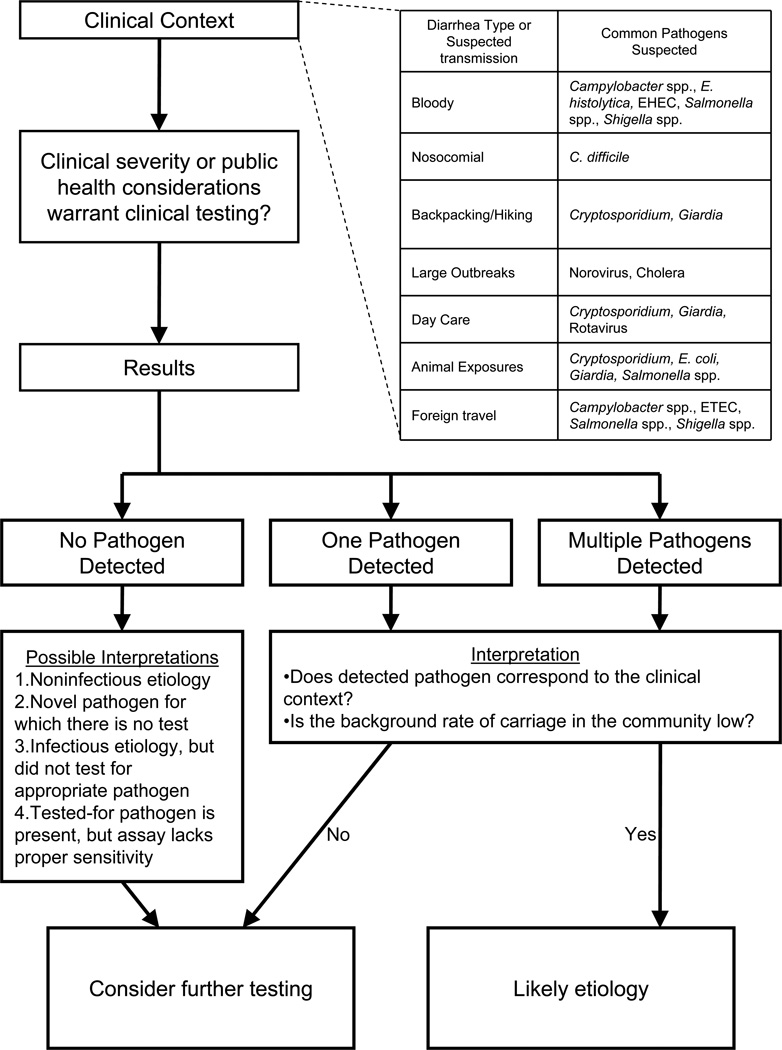
The clinical context of the individual remains critically important before laboratory testing for causes of diarrhea. This is because no matter how perfect the diagnostic test, Bayes’ theorem holds that the posttest probability (e.g., of an infection causing diarrhea) will depend equally on the pretest probability (e.g., clinical likelihood) and the diagnostic test’s performance [4,7].
Upon laboratory testing of stool there are several possible outcomes (Figure 1). The most common result in most settings is that no pathogen is identified. There are several interpretations of this result, highlighted in the figure, one of which is that the pathogen was present in the stool sample but was below the limit of detection of the assay. Stool is a complex matrix of commencal and potentially pathogenic microbes, and finding a rare enteropathogen is inherently challenging. For example, one study estimated as low as 107, and as high as 1010 microbes per gram (dry weight) of stool, depending on the microbial species [8]. In particular, culture for bacteria can be hampered by prior or concurrent use of antimicrobials [9] or overgrowth of commensals. The other possible outcome is that one or more potential pathogens are detected. When this occurs one should always ask what is the background rate of detection of the enteropathogen in the particular setting [10–12], since in many settings enteropathogens can be carried asymptomatically. This background detection rate depends on setting, organism, and test, and is particularly high in children in developing countries and when highly sensitive molecular techniques are used [5]. For example, in a study in Bangladesh, 18% of patients without diarrhea were found to be positive for Giardia by antigen detection [13].
Figure 1.
Understanding the clinical context of an individual remains important in making the decision to perform clinical testing on a stool sample as well as in the proper interpretation of a test result.
Laboratory Methods
While most of this review will focus on newer molecular methods, a brief discussion on traditional culture methods is warranted. Culture protocols involve multiple selective media and reagents, usually for diarrheagenic E. coli, Salmonella, Shigella, and Campylobacter, followed by identification of colonies by appearance, biochemical testing, or molecular probes. Culture is impaired by antibiotic use and is frequently of low yield for identifying an enteropathogen instances [14]. Microscopy is used primarily for parasites, entails concentration methods and staining for morphologic detail, and depends on skilled microscopists. Antigen detection has greatly advanced the detection of enteropathogens by offering stable quality-controlled kits that can be used across laboratories. Such tests are available for protozoa (e.g., Entamoeba histolytica, Cryptosporidium, Giardia), viruses (e.g., rotavirus, astrovirus, adenovirus, norovirus), and bacterial products (e.g., Clostridium difficile toxins, Shiga toxin, Campylobacter). They can be costly and their performance depends on good quality capture and detection antibodies (e.g., low sensitivity for norovirus – [15]).
Nucleic acid amplification techniques for the detection of diarrhea-causing agents have become widespread. Table 1 presents a non-exhaustive list of selected singleplex and multiplexed PCR assays for the detection of diarrheagenic organisms published over the previous several years. We focused on assays that demonstrated sensitivity improvements over conventional assays, highly multiplexed assays for several pathogens, and assays that yielded quantitative results. Most were performed only in research laboratories. These are generally more sensitive than culture, microscopy, or antigen detection as detailed. In one example, a study describing a real-time PCR assay designed to detect E. intestinalis reported a lower limit of detection of as little as 102 spores/mL stool, an improvement over detection by staining and microscopy (≥106 spores/mL stool) [16]. In another example, PCR methods increased the detection of pathogens over conventional methods from 53% to 75% in amongst cases and from 19% to 42% in healthy controls amongst 4,627 samples tested [10]. In a final example, employment of PCR methods resulted in a 22-fold increase in the detection Cryptosporidium and Giardia species versus conventional microscopy [17]. Many are capable of differentiating species (e.g. Cryptosporidium hominis vs. C. parvum) or pathogen subtypes (e.g. diarrheagenic E. coli subtypes) that are impossible to distinguish by morphology. Specificity of PCR is also high, particularly when internal sequence-specific probes are used to confirm detection. These methods are rapid, with the ability to return clinically-relevant results in a matter of hours after nucleic acid extraction.
Table 1.
Selected PCR methods for the detection of diarrheagenic enteropathogens
| Pathogen Type | Pathogen(s) detected | Assay Type(s) | Comments | Reference |
|---|---|---|---|---|
| Bacteria | Vibrio cholera | Conventional, dip stick, PCR, multiplex PCR | Cholera suspect stools did not grow cholera but were PCR positive, potentially due to inactivation by phage. | [18] |
| Clostridium difficile | Cepheid Gene Xpert (PCR), EIA/cytotoxin neutralization | Excellent sensitivity and specificity of PCR vs. the predicate EIA followed by cytotoxin neutralization test | [19] | |
| Campylobacter jejuni; Campylobacter coli | PCR, real-time PCR, commercial EIA | Low sensitivity of culture was between vs. molecular (60% vs. 90%) | [20] | |
| Shiga Toxigenic E. coli | Real-time PCR after enrichment culture | Large public health study showing utility of PCR on submitter broths. | [21] | |
| Diarrheagenic E. coli | Multiplex PCR + Gel analysis | Nine virulence genes targeted over two PCRs and gel analysis; 100% specificity and limit of detection of 104 CFU/mL for tested strains. | [22] | |
| Diarrheagenic E. coli | Multiplex real-time PCR + melt curve analysis | Multiplexed real-time PCR targeting eight virulence genes; reported 99% sensitivity and 100% specificity for recognized diarrheagenic and non-diarrheagenic laboratory strains | [23] | |
| Diarrheagenic E. coli | Multiplex real-time PCR + melt curve analysis | Validation of assay presented in reference [23]; finding of 98% sensitivity and 100% specificity | [24] | |
| Salmonella | Enrichment culture; real-time PCR | Enrichment culture-based PCR was more sensitive than routine bacterial culture alone | [25] | |
| Campylobacter spp. | 16S rRNA PCR | PCR methods used detected Campylobacter in 38% of “no diagnosis” samples | [26] | |
| Diarrheagenic E. coli | Multiplex PCR + Gel analysis | Scheme of 4 multiplexed PCRs to detect 14 E. coli genes. | [27] | |
| Salmonella enterica, Campylobacter jejuni | Real-time PCR | In a prospective study of 2,067 stool samples, use of real-time PCR as a screening method provided a 15% to 18% increase in the pathogen detection rate | [28] | |
| Diarrheagenic E. coli | Multiplex PCR | This multiplex PCR is simultaneously detects six pathotypes of E. coli on pooled isolates. | [29] | |
| Virus | Norovirus GI, GII, and GIV | Real-Time PCR | Quantitative method for all 3 Norovirus genogroups. | [30] |
| Rotavirus A and C, adenovirus, norovirus GI and GII, sapovirus, astrovirus, Aichi virus, parechovirus, enterovirus | Multiplex PCR | Multiplex PCR plus gel method to detect 10 diarrhea-causing viruses. | [31] | |
| Adenovirus, Astrovirus, Norovirus GI and GII, Rotavirus, Sapovirus | Multiplex PCR with bead-based detection | Multiplex PCR plus Luminex-bead based detection method provides similar sensitivity and quantitation as the real-time PCR method. | [32] | |
| Adenovirus, Rotavirus | Real-time PCR, latex agglutination, electron microscopy | PCR methods resulted in increases of 111–175% versus latex agglutination, or electron microscopy. | [33] | |
| Rotavirus | qRT-PCR vs. ELISA | RT-PCR detected several additional infections beyond ELISA however these were subclinical and of low qRT-PCR Ct so a Ct cutoff was recommended. | [34] | |
| Norovirus | qRT-PCR | qRT-PCR Ct cutoff can be used to attribute norovirus to diarrhea cases vs. controls. | [35]* | |
| Norovirus | RT-PCR with HRM | Can distinguish genotype by HRM | [36] | |
| Adenovirus, astrovirus, enterovirus, norovirus, parechovirus, rotavirus, sapovirus | Real-time PCR | Detection of causative agent increased from 49% using conventional methods to 97% using real-time PCR. | [37] | |
| Rotavirus | Real-time PCR | Quantitative real-time PCR was able to detect 28% more rotavirus infections than EIA | [38] | |
| Parasite | Ancyclostoma, Necator americanus, Ascaris lubricoides, Strongyloides stercoralis | Real-time PCR | Detected a pathogen in ~62% of samples vs. ~8% by microscopy | [39] |
| Cryptosporidium spp., Dientamoeba fragilis, Entamoeba histolytica, Giardia intestinalis | Multiplex real-time PCR, singleplex real-time PCR, microscopy | 15% of samples tested were positive under either of the PCR methods whereas only 8% of samples were positive by microscopy. | [40] | |
| Cryptosporidium spp., Giardia intestinalis, Entamoeba histolytica, Ancylostoma duodenale, Ascaris lubricoides, Necator americanus, Strongyloides stercoralis | Multiplex PCR + Bead-based detection | Multiplex assay for seven intestinal parasites offered 83–100% sensitivity/specificity vs. the real time assays. | [41] | |
| Multiple enteropathogens | Campylobacter spp., Salmonella spp., EAEC, EPEC, enterotoxigenic Clostridium perfringens, Cryptosporidium spp., Giardia spp | PCR, microscopy, bacterial cultures, immunoassays | PCR was able to detect a disease agent in 41% of samples compared to only 15% with conventional methods | [17] |
| norovirus, rotavirus, sapovirus, Campylobacter spp., Salmonella spp., EAEC, Cryptosporidium spp., Giardia spp. | Real-time PCR | In 4,627 samples tested, use of PCR increased detection of an enteropathogen versus conventional methods from 53% to 75% in cases and 19% to 42% in controls. | [10]** | |
| Campylobacter jejuni, EIEC, Giardia lambia, Salmonella enteric, Shigella, STEC | Multiplex real-time PCR on broths and direct stool | In screening a total of 28,185 specimens, the pathogen detection rate was 19.2% using MSA versus 6.4% using conventional culture methods. | [42]** |
Note: References in this table are limited to those describing detection of human pathogens
Whether using post-amplification gel-based analysis, or fluorophore-coupled probes (of which there are several varieties) during amplification, the latest generation of PCR-based assays utilize multiplexing capability. Multiplexed PCR testing requires multiple sets of target-specific (i.e. pathogen-specific) primers in the same reaction. In systems utilizing post-amplification gel-based analysis, these multiplexed systems result in identification of pathogens by their amplicon size, such as the assay system presented in Fujioka et al., 2009 [22]. In this work, authors used a pair of multiplexed PCRs coupled to gel analysis to detect nine target genes from the six classes of diarrheagenic E. coli, including stx1, stx2, eaeA, invE, aggR, STh, STp, LT, and astA. In each of the PCRs, primers were placed and amplicons were designed such that the different genes could be distinguished by size. The authors reported a specificity of 100%, with a limit of detection down to 104 CFU/mL for all strains tested amongst 683 E. coli-like isolates tested. A similar system was employed in Rajendran et al., 2010 [27].
Other methods, such as that presented in Guion et al., 2008 [23] and Barletta et al., 2009 [24], use a single PCR reaction to detect eight E. coli virulence genes in a single reaction, with each amplified gene product differing in size. However, instead of employing gel analysis, SYBR Green I was included in the PCR reactions and the amplicons were distinguished by the melt curve analysis of the amplicons. Using this method, authors were able to correctly classify 89 of 90 diarrheagenic E. coli and 36 of 36 non-pathogenic E. coli (sensitivity 99%, specificity 100%). The multiplexed PCR systems described in the above references were employed as a supplement to culture, with the E. coli strains identified from colonies, whereas many other assays described in Table 1 utilize DNA or RNA obtained directly from stool.
While the multiplexed methods presented above are able to overcome the problem of only detecting one pathogen at a time, they are only qualitative in nature. We propose that highly sensitive and qualitative methods may be of great use in predicting the etiologies of diarrhea, operating under the assumption that, especially in cases of mixed pathogen detection, pathogens present at high burden are likely contributors of disease. Addition of sequence-specific, fluorophore-linked probes allows multiplexed PCR systems to become quantitative in nature when standards of known quantities are added to a test run. Use of multiple fluorophores in the same reaction is made possible by thermocycling systems coupled to optical detection packages. The most current real-time PCR thermocyclers are coupled to optical packages that are capable of simultaneously detecting up to six targets through six different emission wavelengths in the same reaction. The paper by de Boer et al., 2010 [42] describes a study in which a pair of multiplexed probe-based real-time PCR reactions to detect Salmonella enterica, Campylobacter jejuni, Giardia lambia, STEC, and EIEC was used as a molecular pre-screening method ahead of culture and microscopy. This molecular screening approach increased the overall pathogen detection rate from 6.4% to 19.2% of samples tested. In an interesting combination of ELISA and quantitative PCR, a study described in Phillips et al., 2009 [34] describes use of ELISA results from well-defined clinical cases and controls to recommend a cut-off value of a quantitative PCR assay results for attributing rotavirus as a cause of infectious intestinal disease. The same group used a similar approach for determining a cut-off in a real-time PCR test for Norovirus [35].
While not discussed in detail for this review, in addition to PCR and real-time PCR, bead-based systems [32,41] as well as microarray systems [43–45] offer simultaneous detection of multiple pathogens through greatly increased multiplexing capability.
While most of the described tests are developed at research laboratories, PCR platforms have become commonplace in reference and clinical laboratories and commercial entities have also developed tests, some of which have been approved by the FDA. The most well known of these are targeted against Clostridium difficile and include the Xpert C. difficile from Cepheid, BD GeneOhm system, and Prodesse ProGastro Cd. In particular, the Xpert C. difficile test has been reported to have a sensitivity of between 41 and 460 CFU/swab [46]. Additionally, commercially developed primers and probes are becoming available, such as those from Fast-track Diagnostics (Luxembourg) and Luminex (xTag Gastrointestinal Pathogen Panel).
There are drawbacks to the multiplexed PCR methods. Creation of an assay panel is not always as simple as combining singleplex tests in the same reaction. One common observation during assay development is that prior to assay optimization, the lower limit of detection for a given analyte in a multiplexed reaction is higher (i.e. the test is less sensitive) as compared to the cognate singleplex test. This can be due to oligonucleotides interacting or interfering with one another. In addition, amplification of multiple targets in a single reaction causes the dNTP pool to be drained faster than reactions with single targets, such that there may not be enough dNTPs late into a reaction to amplify a target of low concentration relative to other targets that are present. This can be especially true of multiplexed PCRs where amplicon sizes are large (≥200nt). While the occurrence of these phenomena vary with each assay or PCR kit used, both oligonucleotide interaction and dNTP drain can potentially become worse as the level of multiplexing goes up, lowering both the sensitivity and reaction efficiency for one or more analytes. Laboratories assembling these types of assays must take these factors into account.
There are also differing costs to implementing these tests. Unmodified oligonucleotide primers are inexpensive and are readily available. SYBR Green and similar intercalating dyes, along with agarose gel systems are also relatively inexpensive. Conversely, use of probe-based real-time PCR drives up costs significantly. This is due to the higher cost of fluorescently-labeled (or otherwise modified) primers and probes, and also the higher cost of thermocycling equipment capable of detecting multiple fluorophores. The cost of the most current real-time PCR cyclers can be as high as $100,000 USD. These costs can affect the availability of these instruments to those laboratories wishing to implement molecular methods.
While these are today’s cutting edge in enteropathogen diagnostics, other technologies are already emerging that could feasibly be in wider use in tomorrow’s laboratories. All-in-one type of solutions (nucleic acid extraction, real-time PCR detection, data analysis) are emerging. Closed multiplexed and arrayed singleplex systems such as the FilmArray from Idaho Technologies and the Taqman Array Card system from Applied Biosystems, respectively, are showing promise for detection of multiple pathogens from a single specimen [47,48]. Ultra high-throughput sequencing, such as is available from 454 Sequencing (Roche) has been used to identify a novel astrovirus during an outbreak of acute gastroenteritis [49,50]. Thus the field of molecular diagnostics is rapidly accelerating.
As for understanding the etiology of diarrhea, we would emphasize that while these new technologies -- particularly highly multiplexed and quantitative approaches -- offer great promise to determining the etiology underlying cases of infectious diarrhea, the increased sensitivity will mean not only increased detection of pathogens in symptomatic cases, but also in non-symptomatic controls. Indeed, in Amar et al., 2007 [10], authors noted that 19% of stool specimens from healthy individuals in this study revealed pathogens when tested with conventional methods, and this increased to 42% of stool specimens when tested with molecular methods. This higher background detection may ultimately lead to the need to quantitate organisms and define a clinical threshold where disease is more likely. Such thresholds are widely used in the viral literature, for instance high plasma viral load of CMV predicts disease [51]. In addition, as diagnostics become more sensitive to detect enteropathogens, the etiology of diarrhea may become obscured by the presence multiple pathogens, and proper research design and attention to the clinical-laboratory context will become more important, not less.
Conclusions
Diarrheal diseases are of enormous global health importance [52,53] and of outbreak significance in developed countries. The variety of organisms known to cause diarrhea presents an inherent challenge to the clinician and the lab. Newer molecular PCR methods have emerged as sensitive, specific, and potentially quantitative tools that will expand our understanding of the etiology of diarrhea when coupled with mindful attention to the clinical setting.
Key Points.
Defining the etiology of any case of diarrhea is complicated by a large list of potential enteropathogens, insensitivity of current conventional methods, occurrence of mixed infections, and appreciable rates of background carriage.
Newer detection methods, especially multiplexed and quantitative PCR, have the capability to enumerate multiple pathogens and define the relative amounts in a single stool sample.
Acknowledgements
This work was supported by NIH U01 AI075396, and the Bill and Melinda Gates Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors declare no conflict of interests.
References
- 1.WHO. Diarrhoeal disease. vol 2011. Geneva: WHO; 2011. Edited by. [Google Scholar]
- 2.Black RE, Cousens S, Johnson HL, J.E. L, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 3.Ricci KA, Girosi F, Tarr PI, Lim YW, Mason C, Miller M, Hughes J, von Seidlein L, Agosti JM, Guerrant RL. Reducing stunting among children: the potential contribution of diagnostics. Nature. 2006;444(Suppl 1):29–38. doi: 10.1038/nature05443. [DOI] [PubMed] [Google Scholar]
- 4.Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, Hennessy T, Griffin PM, DuPont H, Sack RB, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32:331–351. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]
- 5.Fischer Walker CL, Sack D, Black RE. Etiology of diarrhea in older children, adolescents and adults: a systematic review. PLoS Negl Trop Dis. 2010;4:e768. doi: 10.1371/journal.pntd.0000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pawlowski SW, Warren CA, Guerrant R. Diagnosis and treatment of acute or persistent diarrhea. Gastroenterology. 2009;136:1874–1886. doi: 10.1053/j.gastro.2009.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill DR, Beeching NJ. Travelers' diarrhea. Curr Opin Infect Dis. 2010;23:481–487. doi: 10.1097/QCO.0b013e32833dfca5. [DOI] [PubMed] [Google Scholar]
- 8.Franks AH, Harmsen HJ, Raangs GC, Jansen GJ, Schut F, Welling GW. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol. 1998;64:3336–3345. doi: 10.1128/aem.64.9.3336-3345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chitkara YK. Limited value of routine stool cultures in patients receiving antibiotic therapy. Am J Clin Pathol. 2005;123:92–95. doi: 10.1309/eqp21kembb6ehg9b. [DOI] [PubMed] [Google Scholar]
- 10.Amar CF, East CL, Gray J, Iturriza-Gomara M, Maclure EA, McLauchlin J. Detection by PCR of eight groups of enteric pathogens in 4,627 faecal samples: re-examination of the English case-control Infectious Intestinal Disease Study (1993–1996) Eur J Clin Microbiol Infect Dis. 2007;26:311–323. doi: 10.1007/s10096-007-0290-8. [DOI] [PubMed] [Google Scholar]
- 11.Cheun HI, Cho SH, Lee JH, Lim YY, Jeon JH, Yu JR, Kim TS, Lee WJ, Lee DY, Park MS, et al. Infection status of hospitalized diarrheal patients with gastrointestinal protozoa, bacteria, and viruses in the Republic of Korea. Korean J Parasitol. 2010;48:113–120. doi: 10.3347/kjp.2010.48.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen A, Stark K, Kunkel J, Schreier E, Ignatius R, Liesenfeld O, Werber D, Gobel UB, Zeitz M, Schneider T. Aetiology of community-acquired, acute gastroenteritis in hospitalised adults: a prospective cohort study. BMC Infect Dis. 2008;8:143. doi: 10.1186/1471-2334-8-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haque R, Roy S, Kabir M, Stroup SE, Mondal D, Houpt ER. Giardia assemblage A infection and diarrhea in Bangladesh. J Infect Dis. 2005;192:2171–2173. doi: 10.1086/498169. [DOI] [PubMed] [Google Scholar]
- 14.Koplan JP, Fineberg HV, Ferraro MJ, Rosenberg ML. Value of stool cultures. Lancet. 1980;2:413–416. doi: 10.1016/s0140-6736(80)90453-5. [DOI] [PubMed] [Google Scholar]
- 15.Kirby A, Gurgel RQ, Dove W, Vieira SC, Cunliffe NA, Cuevas LE. An evaluation of the RIDASCREEN and IDEIA enzyme immunoassays and the RIDAQUICK immunochromatographic test for the detection of norovirus in faecal specimens. J Clin Virol. 2010;49:254–257. doi: 10.1016/j.jcv.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Wolk DM, Schneider SK, Wengenack NL, Sloan LM, Rosenblatt JE. Real-time PCR method for detection of Encephalitozoon intestinalis from stool specimens. J Clin Microbiol. 2002;40:3922–3928. doi: 10.1128/JCM.40.11.3922-3928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amar CF, East C, Maclure E, McLauchlin J, Jenkins C, Duncanson P, Wareing DR. Blinded application of microscopy, bacteriological culture, immunoassays and PCR to detect gastrointestinal pathogens from faecal samples of patients with community-acquired diarrhoea. Eur J Clin Microbiol Infect Dis. 2004;23:529–534. doi: 10.1007/s10096-004-1149-x. [DOI] [PubMed] [Google Scholar]
- 18.Alam M, Hasan NA, Sultana M, Nair GB, Sadique A, Faruque AS, Endtz HP, Sack RB, Huq A, Colwell RR, et al. Diagnostic limitations to accurate diagnosis of cholera. J Clin Microbiol. 2010;48:3918–3922. doi: 10.1128/JCM.00616-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babady NE, Stiles J, Ruggiero P, Khosa P, Huang D, Shuptar S, Kamboj M, Kiehn TE. Evaluation of the Cepheid Xpert Clostridium difficile Epi assay for diagnosis of Clostridium difficile infection and typing of the NAP1 strain at a cancer hospital. J Clin Microbiol. 2010;48:4519–4524. doi: 10.1128/JCM.01648-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bessede E, Delcamp A, Sifre E, Buissonniere A, Megraud F. New methods for detection of campylobacters in stool samples in comparison to culture. J Clin Microbiol. 2011;49:941–944. doi: 10.1128/JCM.01489-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couturier MR, Lee B, Zelyas N, Chui L. Shiga-toxigenic Escherichia coli detection in stool samples screened for viral gastroenteritis in Alberta, Canada. J Clin Microbiol. 2011;49:574–578. doi: 10.1128/JCM.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujioka M, Kasai K, Miura T, Sato T, Otomo Y. Rapid diagnostic method for the detection of diarrheagenic Escherichia coli by multiplex PCR. Jpn J Infect Dis. 2009;62:476–480. [PubMed] [Google Scholar]
- 23.Guion CE, Ochoa TJ, Walker CM, Barletta F, Cleary TG. Detection of diarrheagenic Escherichia coli by use of melting-curve analysis and real-time multiplex PCR. J Clin Microbiol. 2008;46:1752–1757. doi: 10.1128/JCM.02341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barletta F, Ochoa TJ, Ecker L, Gil AI, Lanata CF, Cleary TG. Validation of five-colony pool analysis using multiplex real-time PCR for detection of diarrheagenic Escherichia coli. J Clin Microbiol. 2009;47:1915–1917. doi: 10.1128/JCM.00608-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin LH, Tsai CY, Hung MH, Fang YT, Ling QD. Rectal swab sampling followed by an enrichment culture-based real-time PCR assay to detect Salmonella enterocolitis in children. Clin Microbiol Infect. 2010 doi: 10.1111/j.1469-0691.2010.03450.x. [DOI] [PubMed] [Google Scholar]
- 26.Maher M, Finnegan C, Collins E, Ward B, Carroll C, Cormican M. Evaluation of culture methods and a DNA probe-based PCR assay for detection of Campylobacter species in clinical specimens of feces. J Clin Microbiol. 2003;41:2980–2986. doi: 10.1128/JCM.41.7.2980-2986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajendran P, Ajjampur SS, Chidambaram D, Chandrabose G, Thangaraj B, Sarkar R, Samuel P, Rajan DP, Kang G. Pathotypes of diarrheagenic Escherichia coli in children attending a tertiary care hospital in South India. Diagn Microbiol Infect Dis. 2010;68:117–122. doi: 10.1016/j.diagmicrobio.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuurman T, de Boer RF, van Zanten E, van Slochteren KR, Scheper HR, Dijk-Alberts BG, Moller AV, Kooistra-Smid AM. Feasibility of a molecular screening method for detection of Salmonella enterica and Campylobacter jejuni in a routine community-based clinical microbiology laboratory. J Clin Microbiol. 2007;45:3692–3700. doi: 10.1128/JCM.00896-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidal M, Kruger E, Duran C, Lagos R, Levine M, Prado V, Toro C, Vidal R. Single multiplex PCR assay to identify simultaneously the six categories of diarrheagenic Escherichia coli associated with enteric infections. J Clin Microbiol. 2005;43:5362–5365. doi: 10.1128/JCM.43.10.5362-5365.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henke-Gendo C, Harste G, Juergens-Saathoff B, Mattner F, Deppe H, Heim A. New real-time PCR detects prolonged norovirus excretion in highly immunosuppressed patients and children. J Clin Microbiol. 2009;47:2855–2862. doi: 10.1128/JCM.00448-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khamrin P, Okame M, Thongprachum A, Nantachit N, Nishimura S, Okitsu S, Maneekarn N, Ushijima H. A single-tube multiplex PCR for rapid detection in feces of 10 viruses causing diarrhea. J Virol Methods. 2011;173:390–393. doi: 10.1016/j.jviromet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Kibiki G, Maro V, Maro A, Kumburu H, Swai N, Taniuchi M, Gratz J, Toney D, Kang G, et al. Multiplex reverse transcription PCR Luminex assay for detection and quantitation of viral agents of gastroenteritis. J Clin Virol. 2011;50:308–313. doi: 10.1016/j.jcv.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logan C, O'Leary JJ, O'Sullivan N. Real-time reverse transcription-PCR for detection of rotavirus and adenovirus as causative agents of acute viral gastroenteritis in children. J Clin Microbiol. 2006;44:3189–3195. doi: 10.1128/JCM.00915-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Phillips G, Lopman B, Tam CC, Iturriza-Gomara M, Brown D, Gray J. Diagnosing rotavirus A associated IID: Using ELISA to identify a cut-off for real time RT-PCR. J Clin Virol. 2009;44:242–245. doi: 10.1016/j.jcv.2008.12.001. *This paper suggests the value of using microbiologically- and clinically-defined sample groups to specify qPCR Ct cutoffs in determining etiology in cases and controls.
- 35.Phillips G, Lopman B, Tam CC, Iturriza-Gomara M, Brown D, Gray J. Diagnosing norovirus-associated infectious intestinal disease using viral load. BMC Infect Dis. 2009;9:63. doi: 10.1186/1471-2334-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tajiri-Utagawa E, Hara M, Takahashi K, Watanabe M, Wakita T. Development of a rapid high-throughput method for high-resolution melting analysis for routine detection and genotyping of noroviruses. J Clin Microbiol. 2009;47:435–440. doi: 10.1128/JCM.01247-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolffs PF, Bruggeman CA, van Well GT, van Loo IH. Replacing Traditional Diagnostics of Fecal Viral Pathogens by a Comprehensive Panel of Real-Time PCRs. J Clin Microbiol. 2011;49:1926–1931. doi: 10.1128/JCM.01925-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng SQ, Halkosalo A, Salminen M, Szakal ED, Puustinen L, Vesikari T. One-step quantitative RT-PCR for the detection of rotavirus in acute gastroenteritis. J Virol Methods. 2008;153:238–240. doi: 10.1016/j.jviromet.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Basuni M, Muhi J, Othman N, Verweij JJ, Ahmad M, Miswan N, Rahumatullah A, Aziz FA, Zainudin NS, Noordin R. A pentaplex real-time polymerase chain reaction assay for detection of four species of soil-transmitted helminths. Am J Trop Med Hyg. 2011;84:338–343. doi: 10.4269/ajtmh.2011.10-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stark D, Al-Qassab SE, Barratt JL, Stanley K, Roberts T, Marriott D, Harkness J, Ellis JT. Evaluation of multiplex tandem real-time PCR for detection of Cryptosporidium spp., Dientamoeba fragilis, Entamoeba histolytica, and Giardia intestinalis in clinical stool samples. J Clin Microbiol. 2011;49:257–262. doi: 10.1128/JCM.01796-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taniuchi M, Verweij JJ, Noor Z, Sobuz SU, Lieshout L, Petri WAJ, Haque R, Houpt ER. High throughput multiplex PCR and probe-based detection with Luminex beads for seven intestinal parasites. Am J Trop Med Hyg. 2011;84:332–337. doi: 10.4269/ajtmh.2011.10-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Boer RF, Ott A, Kesztyus B, Kooistra-Smid AM. Improved detection of five major gastrointestinal pathogens by use of a molecular screening approach. J Clin Microbiol. 2010;48:4140–4146. doi: 10.1128/JCM.01124-10. ** This paper describes an over-three-fold increased pathogen detection rate by combining molecular and conventional approaches, and tested in 28,165 samples.
- 43.Huehn S, Malorny B. DNA microarray for molecular epidemiology of Salmonella. Methods Mol Biol. 2009;551:249–285. doi: 10.1007/978-1-60327-999-4_19. [DOI] [PubMed] [Google Scholar]
- 44.Jin D, Qi H, Chen S, Zeng T, Liu Q, Wang S. Simultaneous detection of six human diarrheal pathogens by using DNA microarray combined with tyramide signal amplification. J Microbiol Methods. 2008;75:365–368. doi: 10.1016/j.mimet.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 45.You Y, Fu C, Zeng X, Fang D, Yan X, Sun B, Xiao D, Zhang J. A novel DNA microarray for rapid diagnosis of enteropathogenic bacteria in stool specimens of patients with diarrhea. J Microbiol Methods. 2008;75:566–571. doi: 10.1016/j.mimet.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Tenover FC, Novak-Weekley S, Woods CW, Peterson LR, Davis T, Schreckenberger P, Fang FC, Dascal A, Gerding DN, Nomura JH, et al. Impact of strain type on detection of toxigenic Clostridium difficile: comparison of molecular diagnostic and enzyme immunoassay approaches. J Clin Microbiol. 2010;48:3719–3724. doi: 10.1128/JCM.00427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rand KH, Rampersaud H, Houck HJ. A Comparison of Two Multiplex Methods for the Detection of Respiratory Viruses: FilmArray RP and xTAG RVP. J Clin Microbiol. 2011 doi: 10.1128/JCM.02582-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kodani M, Yang G, Conklin LM, Travis TC, Whitney CG, Anderson LJ, Schrag SJ, Taylor TH, Jr., Beall BW, Breiman RF, et al. Application of TaqMan(R) Low Density Arrays for Simultaneous Detection of Multiple Respiratory Pathogens. J Clin Microbiol. 2011 doi: 10.1128/JCM.02270-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finkbeiner SR, Li Y, Ruone S, Conrardy C, Gregoricus N, Toney D, Virgin HW, Anderson LJ, Vinje J, Wang D, et al. Identification of a novel astrovirus (astrovirus VA1) associated with an outbreak of acute gastroenteritis. J Virol. 2009;83:10836–10839. doi: 10.1128/JVI.00998-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura S, Yang CS, Sakon N, Ueda M, Tougan T, Yamashita A, Goto N, Takahashi K, Yasunaga T, Ikuta K, et al. Direct metagenomic detection of viral pathogens in nasal and fecal specimens using an unbiased high-throughput sequencing approach. PLoS One. 2009;4:e4219. doi: 10.1371/journal.pone.0004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet. 2000;355:2032–2036. doi: 10.1016/S0140-6736(00)02350-3. [DOI] [PubMed] [Google Scholar]
- 52.Santosham M, Chandran A, Fitzwater S, Fischer-Walker C, Baqui AH, Black R. Progress and barriers for the control of diarrhoeal disease. Lancet. 2010;376:63–67. doi: 10.1016/S0140-6736(10)60356-X. [DOI] [PubMed] [Google Scholar]
- 53.WHO. MDG 4: reduce childhood mortality. vol 2011. Geneva, Switzerland: WHO; 2011. Edited by. [Google Scholar]