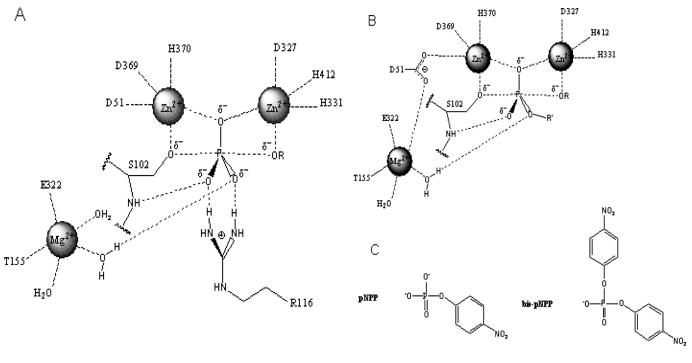
Fig. (2).
Structure of the active site and proposed reaction mechanism of alkaline phosphatase. (A). Schematic illustration of the active site and reaction mechanism of E. coli alkaline phosphatase. The bimetallocentre is occupied by the two Zn2+ ions, and Mg2+ ion at the third site is shown interacting with a non-bridging oxygen via a water molecule. (B). Proposed orientation of the R’ group of the diester substrate in the active site. The R’ group is oriented away from the Mg2+ site suggesting that hydrolysis of the diester substrate is less dependent on rate enhancement contribution from Mg2+. These figures are adapted from Zalatan et al., [5]. (C). Chemical structures of para-nitrophenyl phosphate (pNPP) and bis-para-nitrophenylphosphate (bis-pNPP) used as substrates for the monoesterase and diesterase activities of calf intestinal alkaline phosphatase.