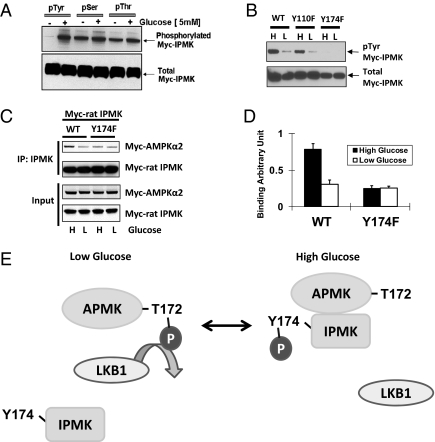
Fig. 4.
IPMK phosphorylation is required for AMPK interaction on the high glucose. (A) High glucose induces IPMK phosphorylation. GT1-7 cells were transfected with Myc rat-IPMK. Cells were starved of glucose for 3 h and resupplied with glucose (5 mM) for 30 min. Phosphorylated protein was immunoprecipitated with phospho-tyrosine, phospho-serine, and phospho-threonine antibodies, respectively. Phosphorylated (top) and total (bottom) IPMK was determined by Western blot. (B) Comparisons of tyrosine phosphorylated IPMK in cells overexpressed WT and tyrosine mutants of rat IPMK. WT or two tyrosine mutants (Y110F and Y174F) were transfected into GT1-7 cells and incubated with DMEM containing high glucose (H) or low glucose (L) for 24 h. Immunoprecipitation was done with phospho-tyrosine antibody to the indicated proteins from lysates and Western blots of tyrosine-phosphorylated IPMK. (C) Myc-AMPKα2 and Myc-IPMK were cotransfected into GT1-7 cells as indicated. Cells were incubated with DMEM containing high glucose (H) or low glucose (L) for 24 h. IPMK was immunoprecipitated, and Western blots were performed. (D) Relative quantifications of bound AMPKα2 and IPMK levels are shown (*Student t test, P < 0.005). (E) Schematic representation of a model explaining how IPMK interacts with AMPK in response to glucose signals. In low glucose, tyrosine phosphorylation of IPMK is decreased, and, subsequently, AMPK can interact with and be phosphorylated by LKB1. In high glucose, increased phosphorylation of Y174 in IPMK interferes with the action of LKB1 to decrease phosphorylation of AMPK. Alternatively, IPMK may promote dephosphorylation of AMPK.