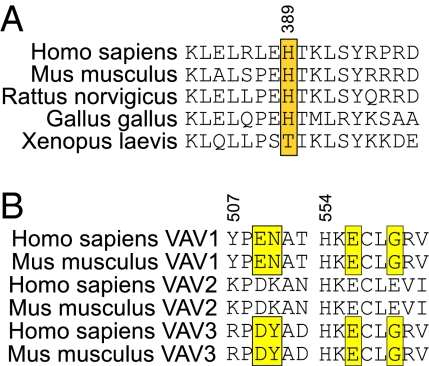
Fig. 6.
Conservation of H389 in NCF2 and its target amino acids in the binding pocket in the ZF domain of Vav. (A) H389 (boxed in yellow) is conserved from Gallus gallus (chicken) to human. (B) Sequence alignment of amino acid residues in the binding pocket in the ZF domain of the three Vav isoforms in the human and mouse are shown. Each human Vav isoform is identical to its relevant mouse homolog. The target amino acids (509, 510, 556, and 559) in Vav1 (yellow) are identical or have conservative substitution in Vav3 (yellow). Replacement of E by D in position 509 is considered a conserved charge; position 510 is less conserved, but the model suggests that NCF2 H389 binds Y510 similar to N510. The conservation of P508 and the general similarity of the character of the residues indicate that this fragment has a similar fold in the three Vav isoforms. The second fragment is even better conserved. E556 is identical in all three variants, and G559 is conserved in Vav1 and Vav3.