Figure 6.
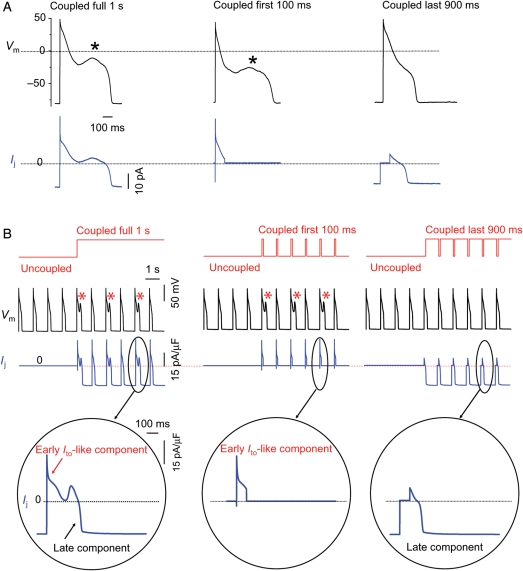
The early Ito-like component of Ij promotes EADs. (A) A patch-clamped myocyte was exposed to 1.0 mmol/L H2O2 to induce bradycardia-dependent EADs at PCL 6 s, which were then suppressed at PCL 1 s (not shown). Coupling the myocyte to a virtual fibroblast (Cf6.3pF, Ef −25 mV, Gj 0.3 nS) (left upper trace) caused EADs to reappear. The virtual gap junction current Ij (left lower trace) consisted of an early transient outward Ito-like component followed by a sustained component. When coupling was allowed only during the first 100 ms of the AP, the EAD persisted (middle upper trace). However, when coupling was allowed only during the last 900 ms, the EAD disappeared (right upper trace). (B) Corresponding simulation: same protocol as in (A), but with the real myocyte replaced by the Luo-Rudy 1 ventricular AP model modified to produce bradycardia-induced EADs. EADs reappear at PCL 1 s when coupling to the fibroblast is present throughout the cardiac cycle (left) or only the first 100 ms of the AP (middle), but not when uncoupled during the first 100 ms of the AP (right). Bottom row shows expanded traces of the corresponding gap junction currents Ij.