Abstract
Introduction
It is still controversial whether borderline lesions with a vulnerable plaque should be stented early or simply treated pharmacologically. No data exist concerning the potential effects of statin therapy on borderline vulnerable lesions in patients with acute coronary syndrome (ACS).
Material and methods
Fifty patients with ACS whose culprit lesions were classified as “borderline lesions” were enrolled. All patients were treated with atorvastatin (20 mg) for 12 months. Intravascular ultrasound (IVUS) was performed and matrix metalloproteinase-9 (MMP-9), tissue inhibitor of metalloproteinase-1 (TIMP-1), and high-sensitive C-reactive protein (hsCRP) levels were measured at baseline and 12-month follow-up.
Results
At 12-month follow-up, we found: 1) IVUS revealed that minimal lumen cross-sectional area (CSA) increased but plaque/media (P&M) area and plaque burden decreased. A total of 25 soft plaques (50%) were transformed into fibrous plaques. 2) ApoB, MMP-9 and hsCRP levels decreased, but TIMP-1 level increased. 3) Stepwise multivariate linear regression analysis showed that the independent predictors for changes in P&M area/year were the decrease in MMP-9 and hsCRP levels.
Conclusions
Atorvastatin therapy stabilized borderline vulnerable plaques and reversed atherosclerosis progression in patients with ACS. Reversal of this progression was accompanied by a decrease in the levels of plasma MMP-9 and hsCRP. Changes in MMP-9 and hsCRP could predict vulnerable plaque stabilization.
Keywords: intravascular ultrasound, atorvastatin, acute coronary syndrome, vulnerable plaque
Introduction
The pathological mechanisms of acute coronary syndrome (ACS) are based on thrombosis secondary to unstable plaque rupture [1]. In many cases, less than 70% of local stenosis of the culprit lesions (lesions directly responsible for the ischaemia episode) is detected by coronary angiography (CAG) [2, 3]. At present, the threshold for clinical revascularization therapy is 70% narrowing [4]. Interventional guidelines of CAD (ACC/AHA 2005) [5] assign those with less than 50% narrowing as Class III, indicating whether angina does or does not exist. It is still controversial whether borderline lesions with a vulnerable plaque should be stented early or only treated pharmacologically. Statins improve the long-term prognosis of patients with coronary artery disease due to plaque stabilization [6]. There are no reports on the frequency of new events caused by progression of borderline lesions in patients with ACS. Evidence suggests that there are no differences between mechanical and pharmacological stabilization of borderline coronary lesions in patients with ACS [7]. The present study prospectively investigates the effect of atorvastatin therapy on borderline vulnerable lesion progression with serial intravascular ultrasound (IVUS) in patients with ACS.
Material and methods
Study population
From May 2007 to December 2008, we enrolled 50 patients with ACS (based on typical symptoms, ECG changes and elevated cardiac troponin T level > 0.1 ng/dl) admitted to the Cardiac Unit in Guangdong Cardiovascular Institute. According to the AHA/ACC guidelines for ACS [5], all patients were treated after admission with antiplatelet and other therapies including angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, β-blockers and statins. Twenty-two of these patients had received statin therapy prior to admission. Atorvastatin (20 mg) was prescribed to all patients as statin therapy for 12 months. Inclusion criteria for these lesions were an angiographic lumen diameter stenosis 50~70% termed borderline lesions, a minimal lumen cross-sectional area ≥ 4.0 mm2 by IVUS, and lesions located in 1 of 3 major epicardial arteries where stent implantation was not performed. When > 1 lesion/patient was studied, the lesion with the larger plaque burden was selected for this study. Culprit lesions were identified by a combination of ECG and CAng. Exclusion criteria included severe calcified lesions and patients with acute ST-elevated acute myocardial infarction (STEMI) where reperfusion strategy is the treatment of choice. Measurements of chemical indicators were altered by acute inflammation or infection, pregnancy or contraindications to therapy with atorvastatin. During the 12-month follow-up, occurrence of major adverse cardiac events, such as acute myocardial infarction (creatine kinase-MB fraction increased to 3 times the upper limit of normal), target lesion revascularization (percutaneous or surgical intervention of lesions), or death from any cause was evaluated. This study was performed with the patients’ written informed consent and approval of the institutional review board. Changes in lipid profiles were calculated at baseline and at 12-month follow-up.
Selective coronary angiography
Selective CAG was performed according to the standard Judkins technique [8]. Before CAG, nitroglycerine (200 µg) was injected into the coronary artery to prevent vessel spasm. Vessel diameters were determined with a 6 F angiographic catheter used as a scaling device. Quantitative coronary analysis was performed using the Digital Cardiac Imaging (DCI) system.
Intravascular ultrasound imaging and analysis
Baseline and 12-month follow-up IVUS examination of culprit lesions were performed in the same manner after intracoronary administration of nitroglycerine (200 µg) with a motorized transducer pull-back system (0.5 mm/s) and a commercial scanner (Boston Scientific Corp./ SCIMED, Natick, Massachusetts), consisting of a rotating 40-MHz transducer with a 2.9 Fr imaging sheath. Intravascular ultrasound images were recorded using the iLab ultrasound imaging system. Quantitative and qualitative analyses were performed according to the criteria of the clinical expert consensus document on IVUS [9] while quantitative IVUS analysis was performed using computerized planimetry (Boston Scientific iReview 1.0 Software). All of these analyses were independently performed by an experienced analyst who was blinded to the lesion and patient background. On playback of the baseline and 12-month follow-up IVUS studies, matching image slices were acquired at 3 different sites of the culprit lesions: the segment with the narrowest lumen cross-sectional area (CSA) and sites 2 mm proximal and distal to the narrowest segment. Quantitative measurements included the external elastic membrane (EEM) CSA, lumen CSA and plaque and media (P&M = external elastic membrane – lumen) CSA. Plaque burden was calculated as the P&M area divided by the EEM CSA. Changes in IVUS measurements between baseline and 12-month follow-up were determined.
Sample measurements
Blood samples were acquired on admission and at the end of follow-up. Commercially available ELISA kits were used for measurement of matrix metalloproteinase-9 (MMP-9; human MMP-9 ELISA, Bender MedSystems Inc), tissue inhibitor of metalloproteinase-1 (TIMP-1) (human TIMP-1 ELISA, Bender MedSystems Inc) and high-sensitive C-reactive protein (hsCRP, hsCRP ELISA, Bender MedSystems Inc).
Statistical analysis
Statistical analyses were performed using SPSS 13.0 software. Data are presented as frequencies or mean ± SD. Comparison was performed with a χ2 test or paired Student’s t test. Multivariate stepwise regression analyses were performed to determine the independent predictors of change in annual changes of P&M cross-sectional area. A p < 0.05 (two-tailed) was considered significant.
Results
Demographic and baseline characteristics
This study involved 50 patients (39 male, 11 female) aged 44-76 years (mean 63.8 ±10.9 years) with ~50 culprit lesions. Hypertension was found in 32 patients (64%) and type 2 diabetes mellitus in 8 patients (16%). Twenty-nine patients (58%) were smokers. Two patients had previous myocardial infarction and 1 patient had a family history of coronary heart disease. Thirty-eight lesions (73.1%) were located at the anterior descending artery, 8 lesions (15.4%) at the circumflex and 6 (11.5%) at the right coronary artery. Two-vessel disease was found in 2 patients.
Lipid profile
Apolipoprotein B (ApoB) levels decreased significantly at follow-up compared with baseline. Levels of other lipoproteins showed minimal change (Table I).
Table I.
Lipid profile at baseline and at follow-up
| TC [mmol/l] | LDL-C [mmol/l] | HDL-C [mmol/l] | TG [mmol/l] | ApoA1 [g/l] | ApoB [g/l] | |
|---|---|---|---|---|---|---|
| Baseline | 4.10 ±0.89 | 1.83 ±0.59 | 1.12 ±0.25 | 1.83 ±1.50 | 1.21 ±0.26 | 0.68 ±0.13 |
| Follow-up | 3.85 ±0.96 | 1.91 ±0.61 | 1.12 ±0.19 | 1.55 ±1.27 | 1.10 ±0.16 | 0.59 ±0.14 |
| Value of p | NS | NS | NS | NS | NS | < 0.05 |
TC – total cholesterol, LDL-C – low-density lipoprotein cholesterol, HDL-C – high-density lipoprotein cholesterol, TG – triglyceride, ApoA1 – apolipoprotein A-I, ApoB – apolipoprotein B
Coronary angiography and intravascular ultrasound data
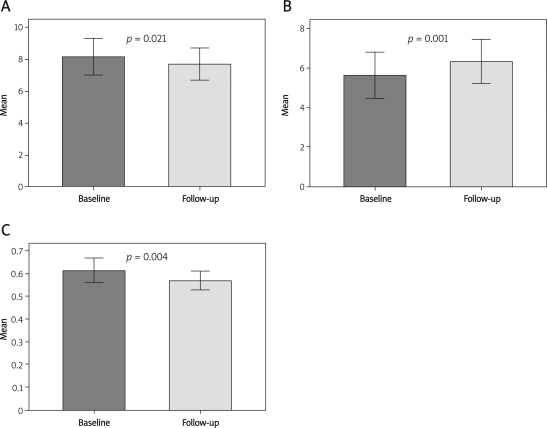
At 12-month follow-up, CAG showed little change in diameter stenosis (62.50 ±10.21% vs. 54.79 ±12.35%, p = 0.48) and area stenosis (58.61 ±8.36% vs. 48.18 ±10.56%, p = 0.78). Minimal lumen CSA increased (p < 0.01), P&M area decreased, and plaque burden decreased (p < 0.01) at 12-month follow-up. Soft plaques transformed into fibrous plaques in 25 cases, indicating stabilization of the plaques (Table II, Figures 1, 2).
Table II.
Intravascular ultrasound imaging at baseline and follow-up
| EEM CSA [mm2] | Minimal lumen CSA [mm2] | P&M area [mm2] | Plaque Burden [%] | Soft plaque n (%) | Fibrous plaque n (%) | Coronary thrombus n (%) | Plaque rupture n (%) | |
|---|---|---|---|---|---|---|---|---|
| Baseline | 13.79 ±3.19 | 5.63 ±2.51 | 8.17 ±2.55 | 61.41 ±10.34 | 43 (86.0%) | 5 (10.0%) | 7 (20%) | 5 (14%) |
| Follow-up | 14.07 ±2.10 | 6.32 ±2.42 | 7.70 ±2.19 | 56.94 ±8.47 | 18 (36.0%) 30 | (60.0%) 0 | (0%) 0 | (0%) |
| Value of p | NS | < 0.01 | < 0.05 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
CSA – cross-sectional area, EEM – external elastic membrane, P&M area – plaque and media cross-sectional area
Figure 1.
A – plaque area change. B – minimal lumen cross-scetional area change. C – plaque burden change at baseline and follow-up
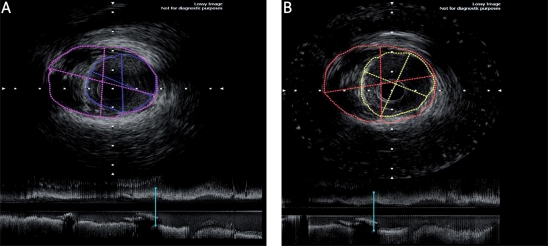
Figure 2.
Representative 3-dimensional intravascular ultrasound analysis at both baseline and follow-up. A – IVUS imaging at baseline. B – IVUS imaging at follow-up
Changes in plasma matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 and high-sensitive C-reactive protein
Plasma MMP-9 and hsCRP decreased significantly, while plasma TIMP-1 levels increased significantly (Table III).
Table III.
Levels of MMP-9, TIMP-1, and hsCRP at baseline and follow-up
| MMP-9 [ng/ml] | TIMP-1 [ng/ml] | hsCRP [mg/l] | |
|---|---|---|---|
| Baseline | 2192 ±881 | 657 ±247 | 3.48±1.50 |
| Follow-up | 1773 ±1085 | 709 ±227 0.39 | 0.39 ±0.19 |
| Value of p | < 0.01 | < 0.01 | < 0.01 |
MMP-9 – matrix metalloproteinase-9, TIMP-1 – tissue inhibitor of metalloproteinase-1, hsCRP – high-sensitive C-reactive protein
Clinical follow-up
There were no adverse events reported during the following-up period.
Relationship between plaque changes and plasma matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 and high-sensitive C-reactive protein levels
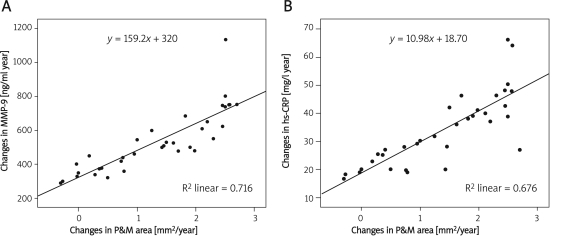
The mean annual change in P&M area was 1.34 ±0.97 mm2/year. Using a stepwise regression model, annual change in MMP-9, annual change in TIMP-1 and annual change in hsCRP were the independent variables, while annual change in P&M area was the dependent variable. The regression equation was: annual change in P&M area = –1.327 ±0.003, annual change in MMP-9 ±0.344, annual change in hsCRP, R2 = 0.830, adjusted R 2 = 0.819, F = 78.152, p = 0.000 (Figure 3).
Figure 3.
A – relationship between plaque changes and changes in the levels of plasma MMP-9. B – relationship between plaque changes and changes in the levels of plasma hs-CRP
Discussion
Although patients with ACS can benefit from revascularization, medical therapy is the basis for their treatment. Atherosclerosis affects not only the culprit lesion but the whole coronary artery tree [10, 11]. As the most important agents in the secondary prevention of coronary artery disease, statins have many pleiotropic effects on the cardiovascular system, including effects on endothelial cells, smooth muscle cells, leukocytes, and platelets [12]. Due to the “pleiotropic” effects of a reduction in inflammation, intensive statin therapy beginning soon after ACS provides a rapid early reduction in clinical events [13]. Many studies have shown that intensive statin therapy could contribute to reversing atherosclerosis progression [14, 15]. By reducing inflammation and improving endothelial function, statins promote plaque stability and prevent ACS [16]. We propose that statins could also stabilize vulnerable lesions in ACS and reverse their progression. This may influence the strategy used in treatment of borderline vulnerable lesions.
In the ESTABLISH [17] trial, a randomized open clinical trial in Japan, 70 patients with ACS received atorvastatin 20 mg daily for 6 months, resulting in a reduction of 13.1% in plaque volume. In contrast, an increase of 8.7% was observed in the control group. The present study also demonstrated that treatment with 20 mg of atorvastatin daily resulted in an increase in minimal lumen CSA as well as a decrease in P&M area. These data support daily administration of 20 mg atorvastatin for inhibition of plaque progression in Asian populations.
The primary goal of lipid-lowing treatment and the importance of low-density lipoprotein cholesterol (LDL-C) as the predictor for coronary events has been emphasized in various guidelines [18, 19]. In the present study, we administered atorvastatin 20 mg daily to patients whose LDL-C levels were at a lower level (LDL-C = 1.83 ±0.59 mmol/l) prior to enrolment. After 12 months on atorvastatin, there was no significant change in the LDL-C level. However, for borderline vulnerable lesions, minimal lumen CSA increased, P&M area decreased, and plaque burden decreased. Furthermore, soft plaques in 25 cases changed to fibrous plaques, indicating stabilization of the plaque. These findings demonstrated that the benefits of atorvastatin therapy for atherosclerosis progression may not be related to LDL-C lowering.
Several studies have focused on lipoproteins other than LDL-C. Long-term prospective studies showed that apolipoprotein A-I (ApoA1), ApoB and the ApoB/ApoA1 ratio correlated with the risk of atherosclerotic cardiovascular disease better than the levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and triglyceride (TG) [20, 21]. Compared with TC/HDL-C and HDL-C/ LDL-C, the ApoB/ApoA1 ratio is a more powerful predictor of risk of a coronary event among untreated patients [22, 23]. For patients who have been previously treated with a statin, LDL-C levels lose their predictive strength but the levels of ApoB and ApoB/A1 work the same way as in untreated patients [23-25].
In the present study, after 12 months on atorvastatin, there were no significant changes in LDL-C. We believe this finding to be attributable to the following factors: 1) 22 patients received statin therapy prior to admission, which was a very high ratio (44%). Since most of these patients’ lipid levels did not change between baseline and follow-up, this high ratio will affect the statistical results even after atorvastatin therapy. 2) The lipid levels of our patients were lower relative to baseline. Therefore, the lipid levels may not change significantly even after atorvastatin therapy at follow-up. 3) The lipid levels of most Chinese patients were low to moderate. However, there was a sharp reduction in ApoB after 12 months. ApoB is found in lipoproteins originating from the [very low-density lipoprotein (VLDL), intermediate density lipoprotein (IDL), LDL] and is the primary apolipoprotein component for lipoproteins with the exception of HDL [26]. ApoB acts as a ligand for lipoprotein receptors in various cells throughout the body, thus playing an important role in atherosclerosis. The significance of the sharp reduction in ApoB in the present study remains unclear. It may suggest a possible reduction in very-low density lipoprotein cholesterol (VLDL-C). Decreases in the level of ApoB may have direct or indirect effects on plaque stability.
Inflammation also plays an important role in ACS progression. Imbalance in the MMPs contributes to plaque instability. Yip et al.[27] and Nomoto et al.[28] demonstrated that an increase in hsCRP is positively related to unstable plaque rupture in local arteries. The present study also showed that the levels of plasma MMP-9 and hsCRP were higher and TIMP-1 was lower following ACS compared with those after 12 months of treatment. Multivariate stepwise regression analyses revealed that decreases in the levels of MMP-9 and hsCRP were positively correlated with the reversion of atherosclerosis progression. The effect of atorvastatin on reversal of atherosclerotic progression was accompanied by changes in the level of inflammatory markers. Therefore, detection of changes in the levels of MMP-9 and hsCRP may provide guidance in monitoring plaque progression and making treatment decisions.
Coronary angiography has long been considered the “gold standard” in diagnosing coronary artery disease [29]. Unlike CAG, which displays the coronary artery as a silhouette of the contrast-filled lumen, IVUS is a new diagnostic technique that provides unique information concerning arterial wall structure and luminal dimensions. Intravascular ultrasound reveals the severity and eccentric nature of the plaque lesions, which may be underestimated in angiographic measurements. Intravascular ultrasound also provides insights into the extent of the lipid-rich plaques, calcification and thickness of the fibrous cap, providing valuable information. De Scheerder et al.[30] found that for borderline lesions detected by CAG, the correlation between IVUS and quantitative CAG data was very weak. Studies by Nishioka et al.[31] and Abizaid et al.[32] supported an IVUS minimum luminal CSA < 4.0 mm2 as a criterion for defining significant stenoses in proximal vessels. Therefore, the present study provided medical therapy for borderline vulnerable lesions without coronary intervention. The ability of atorvastatin to stabilize such lesions defined by CAG and IVUS simultaneously in patients with ACS was confirmed.
Acknowledgments
We declare that we have no financial or personal relationships with other people or organizations that can inappropriately influence our work. There is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript.
References
- 1.Bertrand ME, Simoons ML, Fox KA, et al. Management of acute coronary syndromes acute coronary syndromes without persistent ST segment elevation recommendations of the Task Force of the European Society of Cardiology. Eur Heart J. 2000;21:1406–32. doi: 10.1053/euhj.2000.2301. [DOI] [PubMed] [Google Scholar]
- 2.Dokainish H, Pillai M, Murphy SA, et al. TACTICS-TIMI-18 Investigators. Prognostic implications of elevated troponin in patients with suspected acute coronary syndrome but no critical epicardial coronary disease a TACTICS-TIMI-18 substudy. J Am Coll Cardiol. 2005;45:19–24. doi: 10.1016/j.jacc.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 3.von Korn H, Graefe V, Ohlow MA, et al. Acute coronary syndrome without significant stenosis on angiography characteristics and prognosis. Tex Heart Inst J. 2008;35:406–12. [PMC free article] [PubMed] [Google Scholar]
- 4.Stadius ML, Alderman EL. Coronary artery revascularization Critical need for and consequences of objective angiographic assessment of lesion severity. Circulation. 1990;82:2231–4. doi: 10.1161/01.cir.82.6.2231. [DOI] [PubMed] [Google Scholar]
- 5.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006;113:e166–286. doi: 10.1161/CIRCULATIONAHA.106.173220. [DOI] [PubMed] [Google Scholar]
- 6.Reilly SD, Litovsky SH, Steinkampf MP, Caulfield JB. Statins improve human coronary atherosclerotic plaque morphology. Tex Heart Inst J. 2008;35:99–103. [PMC free article] [PubMed] [Google Scholar]
- 7.Legutko J, Dudek D, Chyrchel M, et al. Safety and effectiveness of pharmacologic versus mechanical stabilization of borderline coronary lesions in patients with acute coronary syndromes. Przegl Lek. 2005;62:1–7. [PubMed] [Google Scholar]
- 8.Norell MS, Perrins J, Meier B, Lincoff AM. Essential interventional cardiology. Philadelphia: Saunders/Elsevier; 2008. pp. 46–9. [Google Scholar]
- 9.Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology clinical expert consensus document on standards for acquisition measurement and reporting of intravascular ultrasound studies (IVUS) a report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2001;37:1478–92. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 10.Mintz GS, Painter JA, Pichard AD, et al. Atherosclerosis in angiographically normal coronary artery reference segments an intravascular ultrasound study with clinical correlations. J Am Coll Cardiol. 1995;25:1479–85. doi: 10.1016/0735-1097(95)00088-l. [DOI] [PubMed] [Google Scholar]
- 11.Casscells W, Naghavi M, Willerson JT. Vulnerable atherosclerotic plaque: a multifocal disease. Circulation. 2003;107:2072–5. doi: 10.1161/01.CIR.0000069329.70061.68. [DOI] [PubMed] [Google Scholar]
- 12.Wang CY, Liao JK. Current advances in statin treatment from molecular mechanisms to clinical practice. Arch Med Sci. 2007;3(Suppl 4A):S91–6. [Google Scholar]
- 13.Kumar A, Cannon CP. The current role of statins in acute coronary syndrome. Arch Med Sci. 2007;3(Suppl 4A):S115–25. [Google Scholar]
- 14.Nissen SE, Nicholls SJ, Sipahi I, et al. ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis the ASTEROID trial. JAMA. 2006;295:1556–65. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 15.Hiro T, Kimura T, Morimoto T, et al. JAPAN-ACS Investigators. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study) J Am Coll Cardiol. 2009;54:293–302. doi: 10.1016/j.jacc.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 16.Sacks FM. Do statins play a role in the early management of the acute coronary syndrome? Eur Heart J Suppl. 2004;6(Suppl A):A32–6. [Google Scholar]
- 17.Okazaki S, Yokoyama T, Miyauchi K, et al. Early statin treatment in patients with acute coronary syndrome demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event the ESTABLISH Study. Circulation. 2004;110:1061–8. doi: 10.1161/01.CIR.0000140261.58966.A4. [DOI] [PubMed] [Google Scholar]
- 18.Brunzell JD, Davidson M, Furberg CD, et al. American Diabetes Association American College of Cardiology Foundation. Lipoprotein management in patients with cardiometabolic risk consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31:811–22. doi: 10.2337/dc08-9018. [DOI] [PubMed] [Google Scholar]
- 19.Maki KC, Davidson MH, Dicklin MR. A comparison of Canadian and American guidelines for lipid management using data from the National Cholesterol Education Program Evaluation ProjecT Utilizing Novel E-technology (NEPTUNE) II survey. Can J Cardiol. 2006;22:315–22. doi: 10.1016/s0828-282x(06)70916-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McQueen MJ, Hawken S, Wang X, et al. INTERHEART study investigators. Lipids lipoproteins and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study) a case-control study. Lancet. 2008;372:224–33. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 21.Panayiotou A, Griffin M, Georgiou N, et al. ApoB/ApoA1 ratio and subclinical atherosclerosis. Int Angiol. 2008;27:74–80. [PubMed] [Google Scholar]
- 22.Walldius G, Jungner I. The apoB/apoA-I ratio a strong new risk factor for cardiovascular disease and a target for lipid-lowering therapy – a review of the evidence. J Intern Med. 2006;259:493–519. doi: 10.1111/j.1365-2796.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 23.Sierra-Johnson J, Fisher RM, Romero-Corral A, et al. Concentration of apolipoprotein B is comparable with the apolipoprotein B/apolipoprotein A-I ratio and better than routine clinical lipid measurements in predicting coronary heart disease mortality findings from a multi-ethnic US population. Eur Heart J. 2009;30:710–7. doi: 10.1093/eurheartj/ehn347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Relationships between lipoprotein components and risk of ischaemic and haemorrhagic stroke in the Apolipoprotein MOrtality RISk study (AMORIS) J Intern Med. 2009;265:275–87. doi: 10.1111/j.1365-2796.2008.02016.x. [DOI] [PubMed] [Google Scholar]
- 25.Danesh J, Erqou S, Walker M, et al. Emerging Risk Factors Collaboration The Emerging Risk Factors Collaboration analysis of individual data on lipid inflammatory and other markers in over 1 1 million participants in 104 prospective studies of cardiovascular diseases. Eur J Epidemiol. 2007;22:839–69. doi: 10.1007/s10654-007-9165-7. [DOI] [PubMed] [Google Scholar]
- 26.Mahley RW, Innerarity TL, Rall SC, Jr, Weisgraber KH. Plasma lipoproteins apolipoprotein structure and function. J Lipid Res. 1984;25:1277–94. [PubMed] [Google Scholar]
- 27.Yip HK, Wu CJ, Hang CL, et al. Levels and values of inflammatory markers in patients with angina pectoris. Int Heart J. 2005;46:571–81. doi: 10.1536/ihj.46.571. [DOI] [PubMed] [Google Scholar]
- 28.Nomoto K, Oguchi S, Watanabe I, Kushiro T, Kanmatsuse K. Involvement of inflammation in acute coronary syndromes assessed by levels of high-sensitivity C-reactive protein matrix metalloproteinase-9 and soluble vascular-cell adhesion molecule-I. J Cardiol. 2003;42:201–6. [PubMed] [Google Scholar]
- 29.Popma JJ. Corornary angiography and intravascular ultrasound imaging. In: Zipes DP, Libby P, Bonow RO, Braunwald E, editors. Braunwald’s heart disease a textbook of cardiovascular medicine. 7th ed. Philadelphia: Elsevier Saunders; 2005. pp. 423–55. [Google Scholar]
- 30.De Scheerder I, De Man F, Herregods MC, et al. Intravascular ultrasound versus angiography for measurement of luminal diameters in normal and diseased coronary arteries. Am Heart J. 1994;127:243–51. doi: 10.1016/0002-8703(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 31.Nishioka T, Amanullah AM, Luo H, et al. Clinical validation of intravascular ultrasound imaging for assessment of coronary stenosis severity comparison with stress myocardial perfusion imaging. J Am Coll Cardiol. 1999;33:1870–8. doi: 10.1016/s0735-1097(99)00100-x. [DOI] [PubMed] [Google Scholar]
- 32.Abizaid A, Pichard AD, Mintz GS, et al. Intravascular-ultrasound-guided percutaneous transluminal coronary angioplasty/provisional stent implantation strategy impact on long-term clinical follow-up. Int J Cardiovasc Intervent. 2001;4:107–14. doi: 10.1080/146288401753258367. [DOI] [PubMed] [Google Scholar]