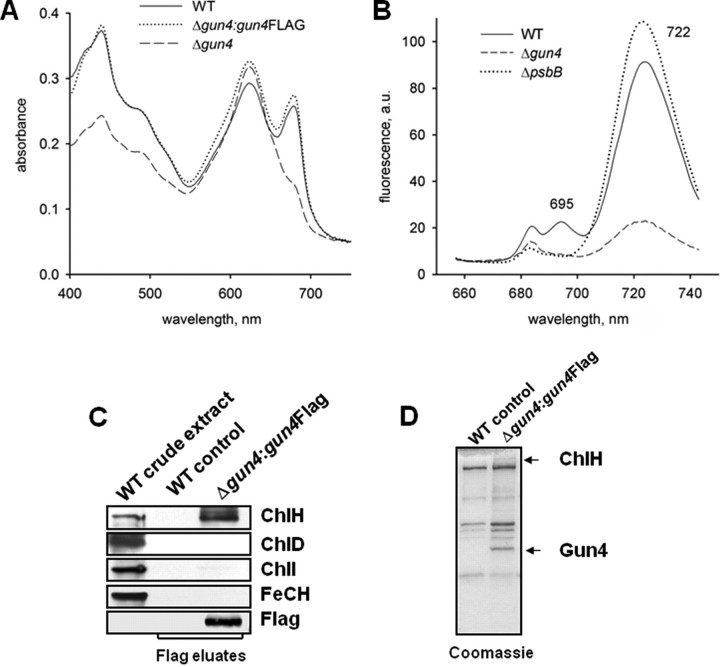
FIGURE 4.
Phenotype of gun4 mutant cells and affinity purification of Gun4. A, absorption spectra of Synechocystis 6803 wild-type (WT) and mutant cells lacking Gun4 (gun4–) or expressing a FLAG-tagged version of Gun4 (Δgun4:gun4FLAG). Cells were grown with 5 mm glucose in the medium. Peaks at 620 and 682 nm represent phycocyanin and Chl absorption, respectively. Spectra of whole cells were measured with a UV-2401 PC spectrophotometer (Shimadzu, Kyoto, Japan). B, semiquantitative 77 K fluorescence emission spectra of thylakoid membranes from Synechocystis 6803 wild-type and mutant cells lacking Gun4 (Δgun4) or CP47 (ΔpsbB). Rhodamine was used as an internal standard, and the spectra were normalized to indicate Chl fluorescence per cell. Peaks at 685/695 and 722 nm represent PSII and PSI complexes, respectively. a.u., absorbance units. C, affinity purification of 3×FLAG-Gun4 and identification of magnesium and ferrochelatase (FeCH) subunits by immunodetection. Solubilized extracts of gun4 mutant cells engineered to express 3×FLAG-Gun4 were immunoprecipitated with anti-FLAG M2-agarose (Sigma-Aldrich). FLAG-Gun4 complexes were eluted with 3×FLAG peptide, resolved by SDS-PAGE, stained with Coomassie (D) or blotted onto a nitrocellulose membrane and immunodetected (C). Wild-type eluate was used as a control.