FIGURE 1.
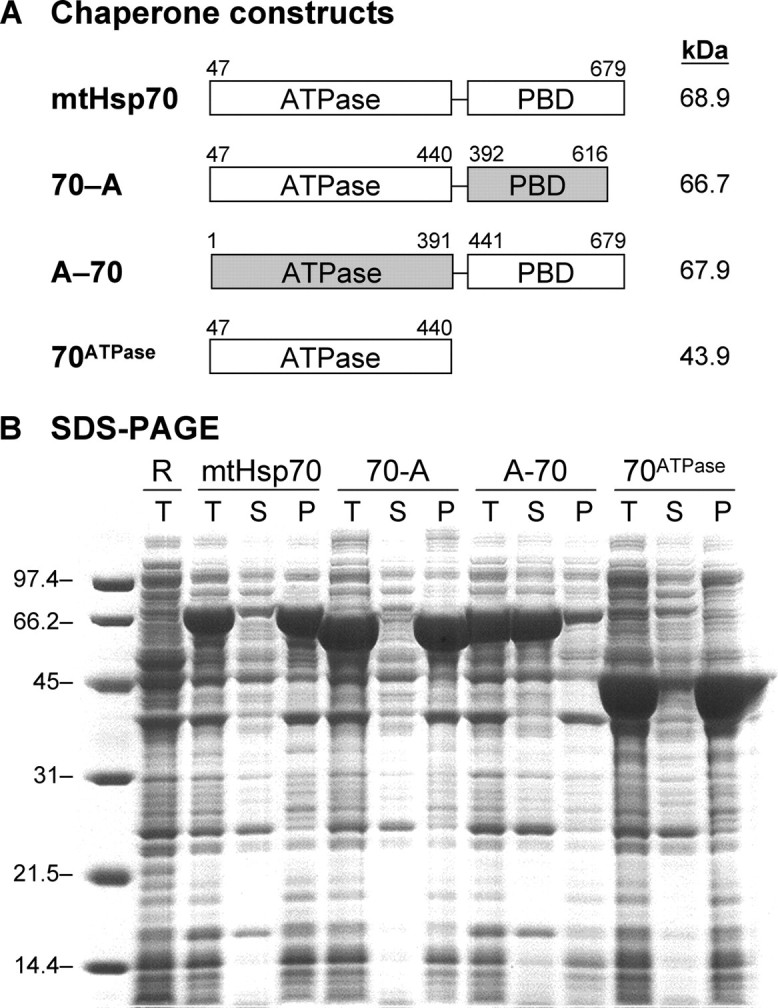
Solubility of mtHsp70, mtHsp70-HscA chimeras, and 70ATPase. A, domain composition of chaperone constructs. B, SDS-PAGE analysis of protein solubility upon expression in E. coli. Molecular weight markers (lane 1), Rosetta 2 E. coli lacking plasmids (lane 2), and cells harboring plasmids for expressing full-length mtHsp70 (pHsp70, lanes 3–5), a chimera having an mtHsp70 ATPase domain and a HscA peptide-binding domain (p70-A, lanes 6–8), a chimera having an HscA ATPase domain and a mtHsp70 PBD (pA-70, lanes 9–11), and the isolated mtHsp70 ATPase domain (pATPase, lanes 12–14). For each sample, 15 μg of total protein from whole cells (T) is shown as well as the soluble lysates (S) and pellets (P) derived from a sample that contained 15 μg of protein before fractionation.
