FIGURE 2.
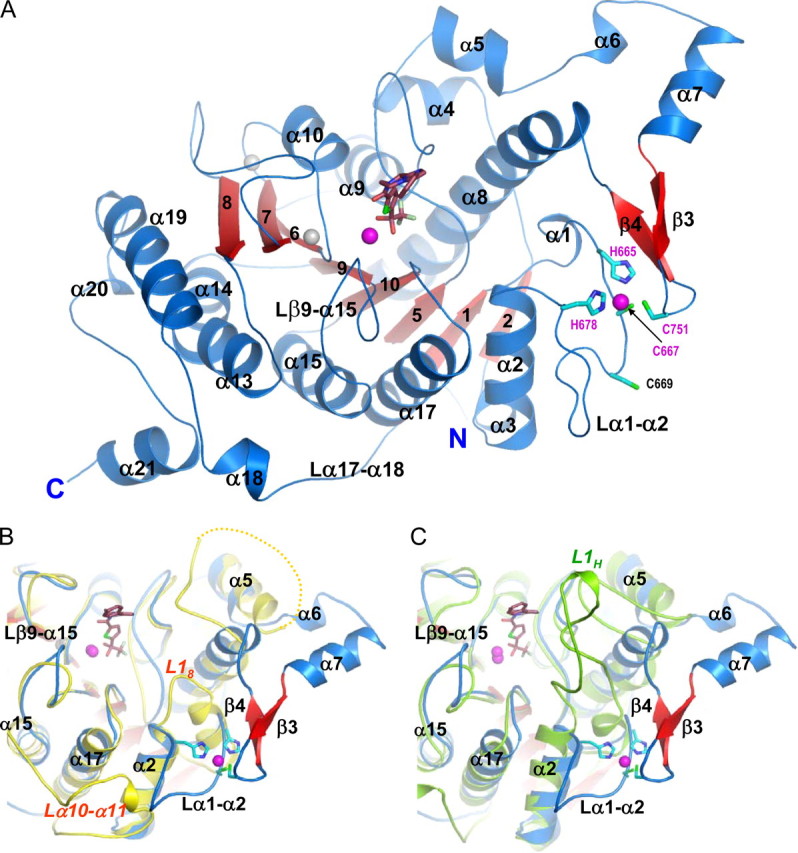
The inhibitor-bound HDAC4 catalytic domain. A, HDAC4cd bound to the TFMK inhibitor (sticks); Protein Data Bank code 2VQJ. Zinc and potassium ions are shown as magenta and gray spheres, respectively. The four residues coordinating the structural zinc ion are shown (sticks). B, a superposition of TFMK-bound HDAC4cd (blue/red) and HDAC8 (yellow). Each α-helix and β-strand of HDAC4 has a structural counterpart in HDAC8, except for α6, α7, β3, β4, and α18. The C terminus of HDAC8 coincides with the end of α19, such that α20 and α21 are also absent from HDAC8. Loop L18 of HDAC8 is much shorter than loop α1-α2 of HDAC4. The yellow dotted line indicates HDAC8 residues missing due to disorder. C, a superposition of TFMK-bound HDAC4cd (blue/red) and HDAH (green). Loop L1H of HDAH is much longer than loop α1-α2 of HDAC4. Both L1H and L18 loops turn inward toward the catalytic site. The major differences with HDAH occur in this loop and in the α6-α7-β3-β4 region and in the longer C terminus of HDAC4.
