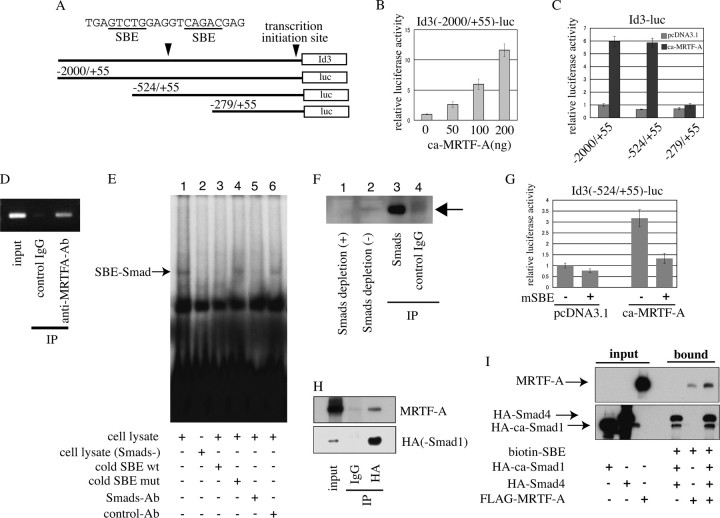
FIGURE 5.
MRTF-A-dependent activation of the Id3 promoter. Transcriptional activity of the Id3 promoter in differentiating myocytes was measured as described under “Experimental Procedures.” A, Id3 promoter constructs used in this analysis are illustrated. The effects of ca-MRTF-A co-expression on the promoter activity of Id3 (2000/+55)-Luc (B), its derivatives (C), and Id3 (-524/+55)-Luc bearing a mutated SBE (G) are shown. The luciferase activities were expressed relative to that in empty plasmid-transfected C2C12 myocytes, which was set as 1.0 (B and G). C, luciferase activity of Id3 (-2000/+55) in cells co-transfected with empty plasmid was set as 1.0. D, ChIP assay shows the interaction of endogenous MRTF-A with the promoter region of the Id3 gene in proliferating C2C12 myoblasts. The extracted chromatin fragments were immunoprecipitated (IP) with the indicated antibodies, and the precipitated genomic DNA was analyzed by PCR using primers for the Id3 promoter region containing the SBE. The size of the PCR product was 216 bp. PCR amplification was also performed prior to immunoprecipitation for the input control. E, 32P-labeled SBE of the Id3 promoter was incubated with the whole-cell extracts of proliferating C2C12 myoblasts (lanes 1 and 3-6) or Smads-depleted whole-cell extracts prepared by pretreatment with anti-Smad1/5/8 antibody (lane 2) in the presence of following additives: 30-fold excess amounts of indicated cold competitors (lanes 3 and 4), an anti-Smad1/5/8 antibody (Ab) (lane 5) or control IgG (lane 6). The reactants were subjected to 5% PAGE. Arrow indicates the SBE-Smad complex. mut, mutant; wt, wild type. F, depletion of Smad1/5/8 in whole-cell extracts of proliferating C2C12 myoblasts was characterized by immunoblotting using anti-Smad1/5/8 antibody. The Smads in C2C12 whole-cell extracts were depleted by immunoprecipitation using anti-Smad1/5/8 antibody. Samples applied to immunoblotting were as follows: Smads-depleted C2C12 whole-cell extracts (lane 1), nondepleted one (lane 2), and immunoprecipitates by anti-Smad1/5/8 antibody (lane 3) or control IgG (lane 4). H, interaction between Smad1 and MRTF-A was analyzed using in vitro-translated proteins. HA-Smad1 and FLAG-MRTF-A proteins were co-incubated, and their interaction was analyzed by immunoprecipitation followed by immunoblotting using the indicated antibodies. I, indicated tagged proteins were translated in vitro and then incubated with a biotinylated-DNA probe containing the SBE of the Id3 promoter. The proteins bound to the probe were collected with streptavidin-conjugated magnetic beads, and the SBE-interacting proteins were analyzed by immunoblotting using the indicated antibodies.