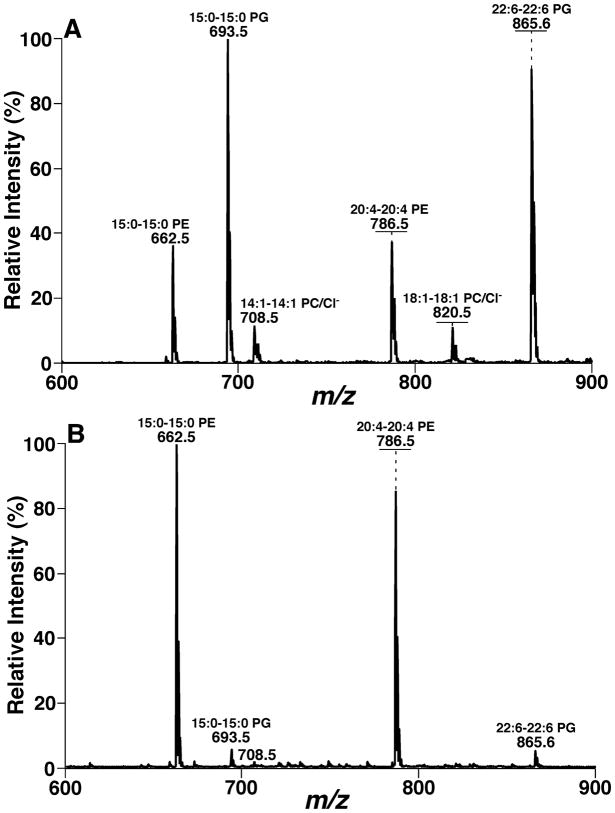
FIGURE 17.
The dependence of ionization efficiency on the charge propensities of analytes. A phospholipid mixture was comprised of 1 pmol/μL each of di15:0 and di22:6 phosphatidylglycerol (PG) (i.e., anionic lipids), 10 pmol/μL each of di14:1 and di18:1 phosphatidylcholine (PC) (i.e., charge neutral lipids), and 15 pmol/μL each of di15:0 and di20:4 phosphatidylethanolamine (PE) (i.e., weakly anionic lipids) species in 1:1 (v/v) chloroform/methanol. The mixture was analyzed with a QqQ mass spectrometer (TSQ Quantum Ultra, Thermo Fisher Scientific) in the negative-ion mode after direct infusion in the absence (Panel A) or presence (Panel B) of 30 pmol/μL of LiOH in methanol. All the indicated molecular species were confirmed with product-ion ESI-MS analysis. The horizontal bars indicate the ion peak intensities after removal of 13C isotopes and normalization of the species to the one with less number of carbon atoms (i.e., the one with lower molecular weight) in each lipid class. (Modified from the ref. (Han et al., 2006b) with permission from the American Society for Mass Spectrometry, Copyright 2006).