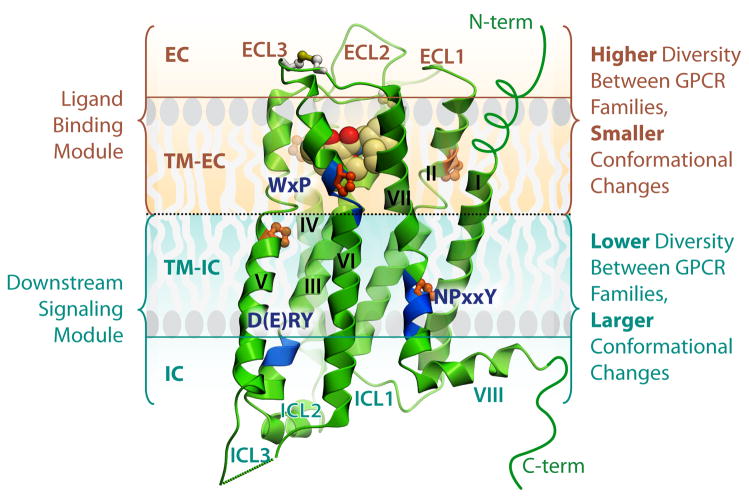
Fig. 2.
General architecture and modularity of GPCRs. Major regions and structural features of GPCRs are shown on example of the D3R crystal structure (PDB ID 3PBL). The EC region includes three ECLs and the N-terminus, which may be represented by a short unstructured peptide (as in most class A GPCRs), or a longer globular-like domain. The 7TM helical bundle contains a number of proline-dependent kinks (prolines shown in orange), that approximately divide the receptor into two modules. The EC module (EC and TM-EC regions) is responsible for binding diverse ligands and has much higher structural diversity. In contrast, the IC module (IC and IC-TM regions), involved in binding downstream effectors including G proteins and arrestins, is more conserved between GPCRs, but undergoes larger conformational changes upon receptor activation. Blue ribbon patches highlight highly conserved, functionally relevant motifs in the TM helices of Class A GPCRs. The C-terminus in most GPCRs is comprised of helix VIII, and in many receptors also has a palmitoylation site anchoring helix VIII to the membrane (not shown).