Abstract
Centenarians represent a rare phenotype appearing in roughly 10–20 per 100,000 persons in most industrialized countries but as high as 40–50 per 100,000 persons in Okinawa, Japan. Siblings of centenarians in Okinawa have been found to have cumulative survival advantages such that female centenarian siblings have a 2.58-fold likelihood and male siblings a 5.43-fold likelihood (versus their birth cohorts) of reaching the age of 90 years. This is indicative of a strong familial component to longevity. Centenarians may live such extraordinarily long lives in large part due to genetic variations that either affect the rate of aging and/or have genes that result in decreased susceptibility to age-associated diseases. Some of the most promising candidate genes appear to be those involved in regulatory pathways such as insulin signaling, immunoinflammatory response, stress resistance or cardiovascular function. Although gene variants with large beneficial effects have been suggested to exist, only APOE, an important regulator of lipoproteins has been consistently associated with a longer human lifespan across numerous populations. As longevity is a very complex trait, several issues challenge our ability to identify its genetic influences, such as control for environmental confounders across time, the lack of precise phenotypes of aging and longevity, statistical power, study design and availability of appropriate study populations. Genetic studies on the Okinawan population suggest that Okinawans are a genetically distinct group that has several characteristics of a founder population, including less genetic diversity, and clustering of specific gene variants, some of which may be related to longevity. Further work on this population and other genetic isolates would be of significant interest to the genetics of human longevity.
Key words: longevity, genetics, centenarians, Okinawa, longevity genes
Introduction
Within the past few generations, both developed and developing countries have witnessed rapid increases in average life expectancy. Initially, this was attributed to large decreases in mortality at younger ages, due mainly to reduction in infant mortality from better public health practices (Vaupel 1997). This was followed by a large reduction in late-life mortality. These changes are unprecedented on an evolutionary time scale and demonstrate significant environmental influences on human lifespan. However, the observation of large variations in the rate of aging and maximum lifespan between species points toward genetic factors as exceedingly important, particularly with regards to determining the essential lifespan potential of a species. In fact, multiple genes in many species have already been shown to affect lifespan. For example, over 70 genes have been found to influence lifespan in the worm model C. elegans (Braeckman and Vanfleteren 2006).
On the one hand, this complexity has led many researchers to conclude that the genetic contribution to human lifespan is far too complex to be meaningfully studied. However, significant extension of both mean and maximum lifespan has already been accomplished through genetic means in lower species including worms, fruit flies and mice (Lin et al. 1998; Guarente and Kenyon 2000; Arantes-Oliveira et al. 2003; Warner 2005). Moreover, this recent progress in identifying longevity-associated genes (hereafter referred to as “longevity genes”) in animal models combined with the advances made through the Human Genome Project and the development of more refined genomic technology should make it possible to identify longevity genes in humans as well.
Extended lifespan, induced by genetic manipulations on less complex organisms, does provide some compelling support for the concept that genes can modify lifespan. The fact that many of these genes are evolutionarily conserved suggests that primitive biological pathways exist from lower organisms to humans that may profoundly impact upon longevity (Barbieri et al. 2003). However, extrapolating results from lower organisms to humans should be done cautiously because of the complexity of human biological and sociocultural systems and because lifespan and causes of mortality in humans are not the same as those in other species. Human populations are characterized by specific gene pools that arise from the unique sociocultural and historical experiences and population movements of particular ethnic groups. Thus, in humans culture and biology are inextricably linked phenomena.
It has been hypothesized that persons who live exceptionally long lives, such as centenarians, do so in large part due to genetic variations that either affect the rate of aging and/or have genes that result in decreased susceptibility to age-associated diseases (Cutler 1975; Schachter 1998; Perls and Terry 2003). For example, it has been found in studies of exceptionally long-lived individuals that many experience delayed onset of major chronic (age-related) diseases until very late in life, often past eighty years of age or later (Evert et al. 2003).
There exist a number of potential genes and regulatory pathways where centenarians may hold a genetic edge over the rest of the population. This article will review some of the most promising candidate genes and pathways that have been found thus far and in the process suggest that centenarians may be promising subjects for future discovery of genetic determinants of human longevity. It will end with a discussion of some of the challenges that currently face researchers and examine different approaches that are currently being undertaken to overcome them. Throughout the article insights from the study of centenarians in Okinawa (Okinawa Centenarian Study) will be highlighted and the unique attractiveness of genetic isolates for the future of genetic investigations will be stressed.
The Okinawa Centenarian Study (OCS): a brief introduction
The OCS is the world’s longest-running population-based study of centenarians. Beginning in 1976 and ongoing, over 900 centenarians have been examined in their own homes, which represents almost a third of all centenarians who have ever existed in the Japanese prefecture of Okinawa. The purpose has been to better understand the genetic and environmental factors contributing to the exceptional longevity of the inhabitants, who are the longest-lived of the Japanese (Sanabe et al. 1977). Although much of the work in the OCS has been cross-sectional and retrospective, its population-based design has helped limit the selection bias towards healthier subjects often seen in other studies of centenarians.
Okinawa also has the highest prevalence of centenarians in Japan despite long-standing socioeconomic disadvantages relative to other Japanese (Cockerham et al. 2000). The high prevalence of very old individuals further suggests that genetics may have played an important role in their survival advantage. However, as of yet, the relative genetic and environmental contributions to the longevity phenotype are still unknown and sociocultural and historical factors are also likely playing an important role.
Okinawans have been the most culturally and geographically isolated of Japanese subpopulations, and they remain resistant to acculturation into the surrounding dominant culture of Japan (Allen 2002). The Okinawans possess a distinctive identity, language, social organization and religion, as well as unique art forms and dietary habits (Lebra 1986; Kerr 2000). Okinawans tend to have large families (highest birthrate within Japan) and relatives often live either in the same household or in nearby cities, towns or villages. Okinawans have traditionally married within their own villages (Lebra 1986; Kerr 2000), increasing the likelihood of a high inbreeding coefficient that may have resulted in clustering of genetic variants affecting health and longevity. Until recently there has been little evidence of substantial gene flow for centuries, resulting in what appears to be less genetic variability in Okinawans than in other Japanese (Tanaka et al. 2004). Genealogical and historical village records are extensive.
The genetic contribution to healthy aging and exceptional longevity has been a primary research area of the OCS. In 1985, in order to assess the genetics of exceptional longevity the first extensive study of centenarian pedigrees was conducted and showed that more long-lived siblings exist in centenarian families (Suzuki et al. 1985) compared to their age matched birth cohort. This work was followed by the first study on the genetics of human longevity using centenarians as a study model, which showed that Okinawan centenarians tended to possess specific type-2 HLA patterns that favor DR1 homozygosity and lower risk for inflammatory disease (Takata et al. 1987). We later replicated this study (Akisaka et al. 1997) and extended the results to other HLA alleles. Some of these findings have been replicated in other populations and inflammation has since become a major focus of studies in CVD and aging (Franceschi et al. 2000; Capri et al. 2006; Candore et al. 2006; Davis and Kipling 2006). The OCS also first reported that cardiovascular health is an important survival factor for centenarians and part of this phenotype includes high HDL levels (Chan et al. 1997a; Suzuki et al. 2001). This finding has been replicated in Ashkenazi Jewish centenarians and at least two genetic variants have recently been found that may offer a partial genetic basis for this phenomenon (Geesaman et al. 2003; Atzmon et al. 2004; Barzilai et al. 2003). Other OCS studies have defined biochemical and hematological factors (Chan et al. 1997b), hormonal patterns (Suzuki and Hirose 1999), measured bone density (Akisaka and Suzuki 1996), characterized nutritional habits (Akisaka et al. 1996; Chan et al. 1997a; Suzuki et al. 2001) assessed cognitive status (Ogura et al. 1995) and verified phenotypic findings with autopsy study (Bernstein et al. 2004).
Discerning true genetic effects from familial patterns in a population is always a challenge due to the fact that families not only share genes in common but families also tend to share common environmental habits. Clearly, some environmental factors do seem to be playing a part in the Okinawan longevity phenomenon—particularly the traditional diet (Willcox 2005). The traditional Okinawan diet includes low-caloric density, plant-based foods such as sweet potatoes, green and yellow vegetables, soy products, fish, and limited amounts of meat (Sho 2001; Suzuki et al. 2001; Willcox et al. 2004). Consistent with their low caloric intake, older Okinawans share several characteristics of the caloric restriction phenotype as part of their exceptional longevity phenotype, including short stature, low body mass index, and high HDL levels relative to other Japanese (Kagawa 1978; Chan et al. 1997a; Willcox BJ et al. 2006).
Interestingly, this phenotype shares several similarities with certain animal models of longevity (Chan et al. 1997b; Willcox DC et al. 2006). For example, several spontaneous or experimentally induced mutations that hinder growth hormone biosynthesis and growth hormone actions, or increase sensitivity to insulin or IGF-1 induce an exceptional longevity phenotype in mice and some other animal models (Bonafe et al. 2003; Bartke et al. 2003). The average lifespan of these mutants increases on the order of 20% to 70% depending on the particular hormonal alterations, gender, diet, and/or the genetic background of the strain. The extended longevity of these mutants is thought to result from lower insulin and IGF-1 levels, higher insulin sensitivity, metabolic changes in carbohydrate and lipid metabolism, reduced production of reactive oxygen species, enhanced antioxidant defenses, greater resistance to cytotoxic stress, and delayed onset of age-related diseases (Barbieri et al. 2003; Tatar et al. 2003; Bartke 2005).
However, a major nutrition transition has taken place in post-war birth cohorts, mainly from the 1960s, and the traditional diet has increased in caloric density with a concomitant mild increase in caloric intake (Todoriki et al. 2004; Willcox 2005). This has been coupled to a decrease in physical activity and the resultant positive energy balance is associated with higher body weight and body mass index in post-World War II cohorts. Yet, the persistence of some of the characteristics of the caloric restriction phenotype in these cohorts, such as shorter stature, and low risk for some chronic age-related diseases, despite the environmental changes, suggests that genetic factors have played an important role in the longevity phenotype in Okinawa (Willcox DC et al. 2006).
Unlike many countries, such as the U.S. in the early 20th century, the current centenarian and near centenarian birth cohorts in Okinawa had relative homogeneity with respect to socioeconomic status and lifestyle. This includes moderate smoking and alcohol consumption, abundant and consistent physical exercise, similar dietary routines, similar access (or lack of access) to healthcare, and a relatively equitable distribution of wealth. This reduces sources of non-genetic variation and makes Okinawa a rare and attractive locale for genetic studies of longevity and healthy aging. For instance, genome-wide association studies may be especially powerful in genetic isolates owing to their increased linkage disequilibrium and decreased allele diversity (Service et al. 2006). The potential and challenges of such studies and other study designs will be discussed in more detail at the end of this article.
Familial clustering of exceptional longevity
From a demographic perspective it seems logical that as the force of mortality increases with advancing age there will be a progressive elimination of those individuals with less favorable genetic polymorphisms (gene variants) so that at exceptional ages (such as centenarians) there comes to exist an enriched gene pool for the study of genes associated with exceptional longevity. However, evolutionary theory posits other mechanisms, such as “antagonistic pleiotropy,” that may actually benefit the health and fitness of younger organisms (of reproducible age) but that can have deleterious effects in older organisms and therefore contribute to aging and risk of age-associated disease (Gavrilov and Gavrilova 2002; Capri et al. 2006).
It has also been hypothesized that genetic polymorphisms may play an even more important role in determining survival at extremely advanced ages. Although family studies have indicated that a modest amount (about 20–30%) of the overall variation in human adult lifespan is due to additive genetic effects it has been unclear whether or not genetic factors become increasingly important for survival at the oldest ages. A recent report suggests that this may indeed be the case. Hjelmborg et al. (2006) studied the genetic influence on human lifespan and how it varies with age using the near extinct cohorts of Danish, Finnish and Swedish twins born between 1870 and 1910 and found that genetic influences on lifespan are minimal prior to age 60 but increase thereafter. In addition, a number of recent studies have also indicated moderate to substantial genetic influence on late-life physical functioning (Christensen et al. 2000; Christensen et al. 2002; Frederiksen and Christensen 2003) as well as cognitive functioning (McClearn et al. 1997; Gatz et al. 2006).
Familial aggregation of longevity has been observed in many diverse populations. For example, in the Utah Population Database, Kerber et al. (2001) found a significant familial relationship in longevity among relatives of long-lived study members including more remotely related members. In Denmark, Frederiksen et al. (2002) found that parental lifespan was positively associated with offspring’s physical and cognitive functioning as well as disease-free survival in a large, cross-sectional population-based survey. Analyzed separately, the effects of the mothers’ and fathers’ ages at death were similar to the combined results. Longer parental survival was associated with reduced odds of having diabetes, hypertension, coronary heart disease (CHD), congestive heart failure, and stroke. Similarly, a relationship between parental longevity and successful aging in elderly males in the U.S. has been reported (Vaillent 1991). Data from centenarian studies in Ashkenazi Jews also has produced similar results (Atzmon et al. 2004). Moreover, most such studies underestimate any real association between parental lifespan and their offspring’s traits because they are cross-sectional and based on interview data, which is more prone to recall bias.
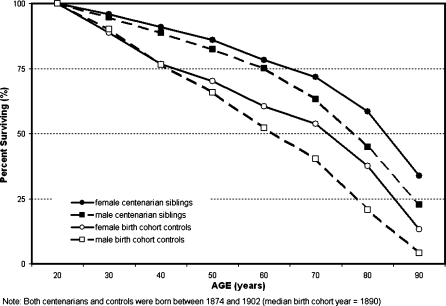
Similar results supporting genetic influences upon longevity have been found for studies of siblings of centenarians. For example, one study found that compared to the rest of the U.S. birth cohort from the year 1900, male siblings of centenarians were at least four times as likely to achieve the age of 90 years and 17 times as likely to attain age 100 or beyond (Perls et al. 1998). We have also found that siblings from our population-based study of centenarians in Okinawa had even more impressive survival rates (Figure 1). We analyzed the pedigrees of 348 centenarian families with 1,142 siblings and compared sibling survival with that of the 1890 Okinawan general population cohort. Both male and female centenarian siblings experienced approximately half the mortality of their birth cohort-matched counterparts (Willcox BJ et al. 2006). This mortality advantage was sustained and did not diminish with age in contrast to many environmentally based mortality gradients, such as education and income. Cumulative survival advantages for this centenarian sibling cohort increased over the lifespan such that female centenarian siblings had a 2.58-fold likelihood and male siblings a 5.43-fold likelihood, versus their birth cohorts, of reaching the age of 90 years. These data support a significant familial component to exceptional human longevity (Willcox BJ et al. 2006).
Figure 1.
Survival of siblings of Okinawan centenarians vs. their respective birth cohorts
Potential longevity enhancing genes and regulatory pathways
Metabolism-related genes and pathways
Studies in model organisms suggest that lifespans may be partially regulated by the insulin/IGF-1 signaling pathway. Growing evidence suggests that disruption of the insulin/IGF-signaling cascade can significantly extend lifespan in diverse species, including yeast, nematodes, fruit flies, and rodents (Longo and Fabrizio 2002; Tatar et al. 2003; Bluher et al. 2003; Warner 2003). For example, model organisms with mutations in the genes daf-2, age-1 and daf-16 in nematodes; sch9 and CYR in yeast; InR, Rpd3, mth, Indy, and chico in fruit flies; and Prop1 and Pit1 in mice all share similar phenotypes, including smaller body size and increased longevity (Clancy et al. 2001; Gems and Partridge 2001; Lin et al. 1998, 2001; Rogina et al. 2000). Other important phenotypic characteristics include reduced insulin signaling, enhanced sensitivity to insulin and reduced plasma levels of insulin-like growth factor-1 (IGF-1). These characteristics, along with subsequent reduced oxidative damage and increased resistance to cytotoxic stress, may be part of an evolutionarily conserved common pathway (Barbieri et al. 2003).
The few human studies on reduced insulin/IGF-1 signaling and human longevity appear to be supportive of findings in animal studies but with some caveats. For example, a case-control study has reported low levels of free plasma IGF in long-lived humans were due to the presence of a specific IGF1-R allele and that different combinations of IGF1-R and P13KCB alleles were shown to influence free-plasma IGF-1 levels as well as longevity (Bonafe et al. 2003). Gene variants causing reduced insulin/IGF-1 signaling (especially insulin signaling component of GH-1) have also been found among long-living Dutch women (van Heemst et al. 2005). However, genetic mutations causing IGF-1 (and GH) deficiencies in humans have also been associated with functional problems and diseases. For example, human gene variants analogous to the prop-1 mutations that extend longevity in mice have been associated with defects that include dwarfism, wrinkled skin and intellectual deficiencies. Interestingly, although few cases have been followed over the lifespan, several of these cases are over the age of 90 years (Longo and Finch 2003).
Reduced levels of plasma glucose and/or insulin/IGF-1 through environmental manipulations such as caloric restriction have also been associated with increased lifespan of numerous species, including humans, and have therefore been suggested as “biomarkers” of aging. For example, the Baltimore Longitudinal Study on Aging noted that healthy men who displayed three “biomarkers of the CR phenotype”—lower insulin levels, lower body temperature and a slower decline in levels of the hormone dehydroepiandrosterone sulfate (DHEA-S) also had significantly longer survival (Roth et al. 2002). Furthermore, similar findings have also been seen in a Japanese American cohort where blood glucose in middle age strongly predicts survival (Rodriguez et al. 1999). Finally, a recent study in Japanese semi-super-centenarians (defined as survival to age 105+ years) examined polymorphic variations in six genes involved in insulin/IGF-1 signaling. This study found one INSR haplotype (composed of two SNPs in linkage disequilibrium) to be more frequent in the centenarians when compared to younger healthy controls (Kojima et al. 2004).
Immuno-inflammatory response genes and pathways
Chronic, low-grade inflammation has been suggested to play an important role in human aging (Franceschi et al. 2000; De Martinis et al. 2005; Capri et al. 2006; Candore et al. 2006; Davis and Kipling 2006; Gupta et al. 2006; Peila and Launer 2006). High circulating levels of cytokines in the blood such as interleukin (IL)-6, C-reactive protein (CRP) and tumor necrosis factor (TNF), among others, have been associated with many age-related diseases, the end result of which is impaired functioning and earlier mortality. Age related increases of IL-6 levels have also been reported, particularly in those elderly undergoing chronic stress (Kiecolt-Glaser et al. 2003); and ‘in vitro’ addition of IL-1 and TNF-α to fibroblasts has been shown to induce an accelerated senescent phenotype (Capri et al. 2006).
Studies have shown that polymorphisms in the IL-6 promoter region appear to affect gene transcription and that a SNP (C174G) has been associated with reduced transcription and improved outcome in a variety of diseases, including several autoimmune conditions, CHD, and some cancers (DeMichele et al. 2003).
On the other hand, studies of centenarians in different geographic locations have been conflicting. Pes et al. (2004) suggested that cytokine-longevity associations may have a population-specific component, being affected by the age and population-specific gene pool as well as by gene-environment interactions. No doubt, one of the main confounding factors in these kinds of case control association studies is undetected differences in genetic background among different populations. To avoid this, Hurme et al. (2005) collected the mortality data of a cohort of 285 nonagenarians (representing mortality between 90 and 95 years of age) and correlated these to the IL-6 genotype. They found that the frequency of -174 allele G was clearly higher in the survivors than in the non-survivors. Although these are exciting findings more research on this polymorphism is needed before any definitive conclusions can be drawn.
Particular alleles of the HLA II locus on chromosome 6 have been shown to vary in prevalence between young and very old members in some ethnic groups suggesting that this locus may have some influence on human longevity. This was also an early area of investigation for the OCS. For example, comparing phenotype frequency of HLA genetic variants in Okinawan centenarians to that of younger controls (mean age of 66 years) revealed that the frequency of HLA-DRw9 was significantly lower but that there was an increased frequency of DR1 (Takata et al. 1987). DRw9 is associated with inflammatory and autoimmune or immune deficiency disease while DR1 seems to offer some protection from these diseases.
Later studies demonstrated that gene frequencies for other HLA alleles also differed between Okinawan centenarians and adult controls from the surrounding areas (Table 1). Allele frequencies of other HLA alleles differed in centenarians compared with those of adult controls who were selected from the same vicinity as the centenarians. The following differences were identified: for the HLA-DQ genes (B1 and A1) the frequencies of DQB1*0503, DQA1*0101 (04) and DQA1*05 were increased in the centenarians, whereas those of DQA1*0102, DQA1*0103 and DQB1*0604 were decreased. Similarly, for the DRB1 gene, the frequencies of DRB1*0101, DRB1*1201 and DRB1*1401 were increased in the centenarians, whereas those of DRB1*0403 and DRB1*1302 were decreased (Akisaka et al. 1997; Akisaka and Suzuki 1998). Therefore, it appears that Okinawan centenarians may be relatively protected from inflammatory and/or autoimmune diseases and that this may be promoting better survival.
Table 1.
HLA, DR and DQ genotype distribution in Okinawans (Takata et al. 1987).
| HLA antigen | Control (n = 159) | Centenarian (n = 82) | p value | ||
|---|---|---|---|---|---|
| n | Frequency (%) | n | Frequency (%) | ||
| DR 1 | 0 | 0 | 5 | 6.1 | 0.0042 |
| DR w9 | 49 | 30.8 | 7 | 8.5 | 0.00004 |
| DR w10 | 0 | 0 | 4 | 4.9 | 0.01276 |
| DQ wd | 135 | 84.9 | 56 | 68.3 | 0.00258 |
However, a recent analysis of HLA-DQA and HLA-DQB frequencies in Sardinian centenarians revealed non-significant differences suggesting only marginal effects of the class II HLA genes on longevity (Scola et al. 2006). A critical reappraisal of the studies on HLA genes, aging and longevity has exposed numerous methodological shortcomings such as insufficient sample sizes, different inclusion criteria and age cut-offs, inappropriate control matching, and neglected consideration of sex-related effects and the different genetic make-up of studied populations among others. Nevertheless, despite these shortcomings it was concluded that there appears to be some HLA gene variants associated with longevity while some of these alleles seem to confer an increased risk for early mortality, depending on life stage (youth, adulthood or older) or sex (Caruso et al. 2000).
Telomere length
Studies have shown that telomeres are associated with cellular senescence and lifespan in model organisms of aging. Telomeres are protective structures at the end of chromosomes that consist of six recurring nucleotide bases (TTAGGG). Small amounts of these terminal sequences are lost with each cell division. The enzyme telomerase compensates for this loss by rebuilding telomeres. The negative correlation between telomere length and the ability to replicate in somatic cells coupled with an observed reduction in telomere length with age, has led to the suggestion that telomeres play a central role in the aging process.
Telomere length is heritable and varies among individuals of the same age and a possible association between telomere length, chronic diseases and late-life mortality has been suggested (Aviv et al. 2006). In the genetic disorder dyskeratosis congenital, telomere shortening is accelerated and patients experience symptoms of premature aging, premature onset of many age-related diseases, as well as early mortality. During the normal aging process, the loss of telomeric DNA in dividing somatic cells may lead to apoptosis, replicative senescence, or neoplastic transformation. Telomeres have been found to be shorter in persons with age associated diseases such as atherosclerosis, vascular dementia, Alzheimer’s disease, osteoporosis, some cancers and even mood disorders, among others (Bekaert et al. 2005; Bisoffi et al. 2006; Edo and Andres 2005; Honig et al. 2006; Panossian et al. 2003; Simon et al. 2006; Samani et al. 2001). Similarly, cross-sectional studies have repeatedly suggested that peripheral blood monocyte telomere length may be a biomarker of aging (Bekaert et al. 2005).
However, not all studies have supported the putative relationship between telomere length and longevity (Bischoff et al. 2006). For example, Martin-Ruiz et al. (2005) measured telomere length in a subset of the Leiden 85-plus study at baseline and after an average time span of between 3.9 and 12.9 years but did not find telomere length at baseline to be predictive for mortality, cardiovascular disease, cancer or dementia. Moreover, in Japanese centenarians, Hirose et al. (1999) did not find telomere length to be correlated with physical functioning (as measured by ADL levels) or cognitive function. Interestingly, they did find that telomere length of female centenarians was longer than that of male centenarians. Currently, it is not known with certainty whether human telomere length is only a proxy for fundamental mechanisms that govern the course of aging or a key factor in its advancement (Aviv 2004).
Mitochondrial gene mutations and reactive oxygen species
Mitochondrial gene variants are among the top candidates for genetic factors that are associated with longer survival. There are several compelling reasons for this. One, mitochondria are the major site of energy production in cells and energy balance has been linked to aging and longevity (Lee et al. 2001). Two, mitochondria are a major source of potential radical-induced cellular damage. Three, mitochondrial DNA and other subcomponents, due to their close proximity to the site of radical production, are at higher risk of damage and subsequent dysfunction.
Genetic variation in mtDNA may lead to differences both in the functioning of the respiratory chain and in free radical production. In somatic cells, mitochondrial DNA (mtDNA) mutates at up to 20 times the rate of nuclear DNA (Merriwether et al. 1991) and these mutations accumulate with age. More than 250 pathogenic point mutations and rearrangements of the mitochondrial genome have been reported in a spectrum of clinical disorders, which exhibit prominent muscle and central nervous system involvement (Greaves and Taylor 2006). The degree of age-related mutations in mtDNA also varies between people and may be associated with human aging (Chinnery et al. 2002).
There are only a few major haplotypes of mitochondrial DNA in humans and an association between certain mtDNA haplogroups and longevity has been proposed. For example, Niemi et al. (2003, 2005) studied the frequencies of mtDNA haplogroups and haplogroup clusters among elderly subjects and controls in a Finnish population and found that haplogroups U and J were more frequent among nonagenarians. An Italian study of centenarians and younger controls also found that the J haplogroup was notably higher among male centenarians from the Northern region but a later Italian study failed to confirm this finding suggesting that the original findings were population specific (De Benedictis et al. 1999; Dato et al. 2004). Zhang et al. (2003) found a strikingly higher frequency of the C150T mutation in the replication control region of leukocytic mtDNA in Italian centenarians. In another study, the 150T mutation was also found to be more frequent among the very old in both Finnish and Japanese subjects (Niemi et al. 2005). Interestingly, the association was not similar in all haplogroups, and a stratified analysis revealed that two additional common polymorphisms, 489C and 10398G, modified the association between 150T and longevity. The authors suggest that longevity is partly determined by epistatic interactions involving these three mtDNA loci.
Tanaka et al. (2000) analyzed the mtDNA from Japanese centenarians and identified a longevity-associated mitochondrial genotype, Mt5178A that has been associated with less frequent occurrence of mtDNA mutations in the oocytes as well as possibly a deceleration of the accumulation of mtDNA mutations in the somatic cells with increasing age. The authors concluded that this genotype is likely to confer resistance to adult-onset diseases. More research is needed to confirm these preliminary studies but they suggest that certain haplotypes are associated with increased lifespan because they either modulate energy metabolism and oxidative damage and/or the rate of mtDNA mutation. Moreover, recent research has suggested that interactions between mtDNA and nuclear DNA variability occur in humans that can be interpreted as an “ancestral mtDNA/nDNA cross-talk” that affects aging and longevity (De Benedictis and Franceschi 2006).
Stress resistance genes and pathways
Emerging findings suggest that many of the environmental factors that might promote healthy aging, such as caloric restriction or exercise, exert their effects through a hormesis-like mechanism (Masoro 2006). Cells possess a variety of stress response signaling pathways that induce the expression of genes that encode protective proteins such as heat shock proteins, growth factors and antioxidant enzymes. Animal findings support this line of reasoning by showing that stress resistance at the cellular level (resistance to high temperatures, starvation, and damage from reactive oxygen species or ROS) correlates with length of life at the level of the organism (Lithgow and Walker 2002). Several of these genes have human homologs.
Although the genes involved in stress resistance have yet to be explored in sufficient detail to make definitive conclusions regarding longevity related polymorphisms, some of the most promising appear to be related to the super oxide dismutases (SOD). Superoxide is among the most abundant ROS produced by the mitochondria. There is increasing evidence that ROS are not only toxic but also play an important role in cellular signaling and in the regulation of gene expression (Ishii et al. 2006). Superoxide and other ROS can damage cellular machinery and levels of oxidative damage products have been positively correlated with characteristic features of aging (Sampayo et al. 2003). SOD enzymes catalyze the breakdown of superoxide into hydrogen peroxide and water and are therefore central regulators of ROS levels.
Deleterious mutations in these genes have been found to be associated with neurological diseases such as amyotrophic lateral sclerosis (Turner et al. 2005; Julien and Kriz 2006) but the current literature is controversial with regard to polymorphisms that lower risk for morbidity or that are associated with extended lifespan. For example, De Benedictis et al. (1998) did not find any age-dependent difference in the allelic or gentotypic frequency regarding SOD2 polymorphisms (T/C 401nt) in Italian centenarians compared to younger subjects matched for sex and geographic area. However, more recent research supports the potential role of the Ala16Val polymorphism in human aging with the AA genotype associated with increased risk for prostate and breast cancer, immunosenescence profile, as well as DNA damage (Taufer et al. 2005). Other studies have found MnSOD genotypes may modify individual cancer risk (Ambrosone et al. 1999; Mitrunen et al. 2001; Stoehlmacher et al. 2002; Woodson et al. 2003). Nevertheless not all studies support this putative association (Egan et al. 2003; Lin et al. 2003; Hung et al. 2004).
Regulation of genome maintenance and repair
DNA damage accumulates with age, and defects in genes responsible for DNA repair can lead to phenotypes resembling accelerated aging states such as Ataxia-telangiectasia (ATM), Dyskeratosis congenital (DKC1), Hutchinson-Gilford Progeria Syndrome (LMNA), Rothmund–Thomson Syndrome (RECQL4), and Werner Syndrome (WRN) although they are not completely synonymous with the aging process (Martin et al. 1999).
Hutchinson-Gilford Progeria Syndrome (HGPS), for example, is characterized by hair loss, growth retardation, lack of subcutaneous fat, wrinkled (aged-looking) skin, osteoporosis, and arteriosclerosis with patients usually dying from cardiovascular disease before the age of twenty (Pollex and Hegele 2004; Hennekam 2006). Alzheimer-type dementia is not observed nor are other markers of aging such as intracellular deposits of lipofuscins. The most common form of HGPS is caused by a mutation in the human nuclear lamin A gene (LMNA) which codes for a group of proteins called lamins, which maintain the mechanical properties and shape of nuclei. Lamins are also distributed throughout the nucleoplasm, where they appear to be essential for DNA replication and RNA polymerase II transcription. Interest in the lamins has increased because mutations or incorrect processing cause more than a dozen different inherited diseases, ranging from striated muscular diseases, via fat and peripheral nerve cell diseases, to progeria (Broers et al. 2006). The effects of less severe LMNA genetic variants on extended survival remain to be explored.
The Werner syndrome gene (WRN) encodes a novel helicase of 1,432 amino acids. Homozygous mutations, all of which result in the truncation of the protein, lead to Werner syndrome. However, little is known about the role of WRN in so-called normal aging. Some SNPs seem to be protective against age-related diseases. For example, the 1367 Arg allele of the WRN gene has been found to be associated with reduced risk for type 2 diabetes (Hirai et al. 2005) and myocardial infarction (Ye et al. 1997) in Japanese subjects. However, Castro et al. (1999) compared the frequency of the C1367A polymorphism in centenarians to that of newborns within the Finnish population, and found no differences in the proportions of 1367 Cys/Arg across age groups. These findings suggest no evidence of an association of this WRN polymorphism with longevity, at least in this population.
It has been suggested that at least some aspects of normal aging are the consequence of anti-cancer mechanisms designed to deal with damaged DNA pathways including those that reduce DNA damage levels from exogenous sources, replication errors and by-products of cellular respiration (Mitchell et al. 2003; Campisi 2003). As unrepaired DNA damage leads to permanent changes in the genetic code that may be oncogenic, pathways that repair DNA damage are important anti-cancer mechanisms. In addition, there are anti-cancer pathways that respond to DNA damage by either preventing cellular replication or inducing cell death. Genes in these pathways code for proteins that reduce cancer incidence. Data from mouse models, many that were originally designed to study cancer, suggest that a potential consequence of DNA damage and response to DNA damage may be aging (Hasty 2005).
Genes and pathways affecting cardiovascular disease
Avoidance or delayed onset of CHD and a better cardiovascular disease risk factor profile are important familial aspects of centenarian survival. For example, individuals with exceptional longevity and their offspring have been shown to have significantly larger high-density lipoproteins (HDL) and low-density lipoprotein (LDL) particle sizes, which confers protection against coronary heart disease (Barzilai et al. 2003). These individuals also had lower prevalence of hypertension, stroke, and metabolic syndrome. These phenotypes were associated with increased homozygosity for the 405 valine allele (VV genotype) in the cholesterol ester transfer protein (CETP) gene compared to controls. The authors hypothesized that lipoprotein particle sizes are heritable and larger particle sizes were associated with delayed aging. However, CETP deficiency and other polymorphisms in CETP were found to have no association with longevity in a study of Japanese centenarians, perhaps due to low CHD risk in Japanese (Arai et al. 2003). Recent prospective analyses of a Japanese American male cohort from the Honolulu Heart Program, where CHD is more prevalent than in Japan, revealed a trend towards decreased CHD in the small number of men with the same intron 14 splicing defect (Curb et al. 2004).
A positional candidate gene for longevity identified from a genome-wide linkage scan among long-lived families in the US by Puca et al. (2001) involved in lipoprotein metabolism, microsomal triglyceride transfer protein (MTTP) was found to be suggestive of a link to human longevity. Two single nucleotide polymorphisms (SNPs) have been found to account for most of the variation at the MTTP locus and a haplotype that contains both of these was found to be significantly less frequent in long-lived individuals compared to younger controls (Geesaman et al. 2003). However, this finding has not been supported in other ethnic groups (Beekman et al. 2006).
An insertion/deletion variant in the gene encoding ACE (angiotensin I-converting enzyme) has also been proposed to have links to longevity as an increased frequency of homozygotes for the D (deletion) allele was found in German octogenarians and these results were supported in a longitudinal (follow-up) study of Danish twins (Luft 1999; Frederiksen et al. 2003). Polymorphisms in ACE have been suggested to be involved in cardiovascular and renal diseases due to negative effects associated with vasoconstriction. However, two large studies of centenarians and younger controls failed to replicate these findings (Bladbjerg et al. 1999; Blanche et al. 2001).
In contrast to almost all of the other candidate genes reviewed so far, cross-sectional analyses of apolipoprotein E (APOE) allele frequencies among different age groups have been strikingly consistent. The epsilon4 (APOE4) allele (deleterious type) among centenarians has been found to be about half as common as that of younger adults in numerous different ethnic groups (Christensen et al. 2006). APOE plays an important role in regulating lipoproteins and is found in three forms: APOE2, APOE3 and APOE4. APOE4 has been consistently associated with a moderately increased risk of both cardiovascular disease and Alzheimer disease while APOE2 seems to confer protection. Risk also seems to increase for APOE4 carriers with negative environmental exposures such as head trauma, diabetes, atherosclerosis, or peripheral vascular disease, since these phenotypes are at even higher risk of cognitive decline (Jordan et al. 1997; Haan et al. 1999).
Table 2 outlines several genetic variants and associated biological pathways that have been implicated in human longevity, many of these genetic variants have been identified from centenarian studies.
Table 2.
Selected candidate genes and pathways that may be related to human longevity.
| Gene | Sample | Finding |
|---|---|---|
| PATHWAY: Metabolism: Growth hormone/IGF-1 axis | ||
| IGF-1 | Dutch adults (Rotterdam study) | Noncarriers of the 192-bp allele in promoter had increased relative risk for Type 2 diabetes and myocardial infarction (Vaessen et al. 2001) |
| Elderly Dutch women (Rotterdam study) | Baseline bone mineral density lower and bone loss higher in noncarriers of the 192-bp allele (Rivadeneira et al. 2003) | |
| KLOTHO | Czechs, U.S. Caucasians, and U.S. African Americans | KL-VS homozygotes less prevalent in the elderly (Arking et al. 2002) |
| PATHWAY: Inflammation | ||
| HLA | Okinawans aged 90+ and adult controls | HLA-DR9 allele under-represented and HLA-DR1 allele over-represented in long-lived Okinawans (Takata et al. 1987; Akisaka et al. 1997) |
| IL-6 | Octo/nonagenarians vs. younger controls (Belfast study) | Frequency of IL-6-174 G/G decreases with age (Rea et al. 2003) |
| Finnish nonagenarians vs. healthy blood donors, aged 18–60 | No statistically significant differences in IL-6-174 G/G genotype distributions (Wang et al. 2001) | |
| PATHWAY: Mitochondrial energy production | ||
| Mt5178A | Japanese centenarians | More frequent in centenarians and may be related to increased resistance to age associated disease (Tanaka et al. 2000) |
| PATHWAY: Stress resistance | ||
| HSP70-1 | Italians aged 18–109 | Age-related decrease of the allele A frequency in −110A > C promoter region polymorphism in females (Altomare et al. 2003) |
| Danish septuagenarian twins | Association between low self-rated health and heterozygosity for −110A > C polymorphism (Singh et al. 2004) | |
| SIRT3 | Italian males, young and old | G477T genotype TT decreases while GT genotype increases in the elderly (Rose et al. 2003) |
| PATHWAY: Genome maintenance and repair | ||
| WRN | Finnish newborns, Finnish centenarians, Mexican newborns | Decline of 1074Phe/Phe genotype of 1074Leu/Phe polymorphism with age (Castro et al. 2000) |
| PATHWAY: Cardiovascular | ||
| ACE | French centenarians vs. adults, aged 20–70 | II genotype is more frequent in centenarians (Schachter et al. 1994) |
| Danish twins, aged 73 + | Relative risk of death increased in II genotype (Frederiksen et al. 2003) | |
| ApoE | French centenarians vs. adults, aged 20–70 | ɛ4 allele less frequent in centenarians than controls; 2 allele reciprocally increased (Schachter et al. 1994) |
| U.S. aged 90+ vs. young adults, aged 18–25 | Same as above (Zubenko et al. 2002) | |
| Danish centenarians and male controls, age 40 | Average relative mortality risk was lower for ɛ2 allele (Gerdes et al. 2000) | |
| CETP | Ashkenazi Jewish aged 95+ and their offspring vs. adult controls | Probands and offspring had increased homozygosity for VV genotype (I-405-V) (Barzilai et al. 2003) |
| Japanese centenarians and healthy younger controls | No association with longevity (Arai et al. 2003) | |
| 7 years prospective follow-up of Japanese American men aged 71–93 | Borderline reduced risk for heart disease (Curb et al. 2004) | |
| MTTP | American centenarians and long-lived siblings | Minor allele rs 2866164 (allele G) under-represented (Geesaman et al. 2003) |
| Germans aged 90+ and adult controls | No differences (Nebel et al. 2005) | |
| PON I | Italian centenarians vs. younger controls, aged forties | Significant difference in haplotype 192R/55L frequency between older and younger individuals (Rea et al. 2004) |
| Irish octo/nonagenarians vs. adolescents | ||
| MTHFR | Dutch elderly, aged 85+, vs. younger controls, aged 18–40 | 677T/T (Val/Val) genotype less prevalent in elderly men (Heijmans et al. 2000) |
| Swiss, healthy younger and older subjects | 677T allele was 1.4 times less frequent in older individuals (Todesco et al. 1999) | |
The genetic epidemiology of exceptional longevity: challenges and opportunities
From the previous discussion it seems clear that the length of the human lifespan appears to be controlled by a complex interaction of genetic, epigenetic and environmental factors. As has been observed for other complex traits, genetic control of the human lifespan is also likely to be determined by subtle variations in numerous “hub genes” that participate in multiple regulatory pathways, resulting in weak to moderate effects. The complexity of this phenotype is a challenge that has likely been slowing progress in this area. In order to identify longevity genes in human beings there are a number of different strategies available. The merits and demerits of some of the most common strategies are discussed below.
Approaches to identifying human longevity genes
In order to identify genes linked to a particular phenotype in humans, two types of approaches are commonly used: linkage approaches and population-based association studies, both of which are being extended to human longevity research (Nebel and Schreiber 2005). Linkage analysis studies, in which gene loci can be found by co-segregation of a polymorphic marker allele with the phenotype under study, are particularly useful when one also has extended pedigrees from study subjects, which is rarely the case. However, linkage approaches using small core pedigrees or with long-lived sibpairs can also be performed. In the case of sibpairs, a greater than expected proportion of shared alleles between two sibs with the same phenotype indicates that a gene involved with this phenotype is located in a nearby region. Linkage analysis is not subject to confounding due to population stratification (differences in allele frequencies between subpopulations due to differences in ethnicity, geography, or historical population movements).
However, linkage studies usually can only detect loci with relatively strong effects. To achieve the statistical power needed for identification of the weak or moderate susceptibility factors that are likely to play a role in human longevity, DNA samples from a very large sample of long-lived individuals is required, as in the GEHA project (http://www.geha.unibo.it) which is collecting 2,650 long-lived (aged 90+) sibpairs from 11 European countries or the Long-life Family Study which also studies large numbers of long-lived families (http://www.biostat.wustl.edu/llfs). Another disadvantage of linkage analysis is that the identified chromosomal candidate regions are usually large and have to be narrowed by subsequent association and linkage disequilibrium studies in order to be able to localize specific genes.
Recent advances in ultra-high-throughput genotyping technology and statistical analysis have made population-based association studies, such as case-control studies, an attractive alternative. Although complete genome sequencing is not yet feasible for large studies, it is now possible to type a large number of common SNPs, which account for the majority of genetic variation in human genomes (Hirschhorn and Daly 2005). Association studies, compared to linkage studies, provide more precise localization of susceptibility genes, often extending to only a few thousand base pairs.
Future investigations are increasingly turning to large-scale case-control studies in which entire genomes (or candidate genes representing functional pathways) are assessed for sequence variation and for association with longevity phenotypes. However, in order to avoid population stratification issues, case-control studies require that cases and controls be sampled from the same population (Nebel et al. 2005; Hirschhorn and Daly 2005). Thus, the availability of large and appropriate study samples remains a substantial obstacle.
Study designs
Centenarians as representative of the longevity phenotype
Centenarians represent a rare phenotype appearing in roughly 10–20 per 100,000 persons in most industrialized countries but as high as 40–50 per 100,000 persons in Okinawa, Japan (Willcox DC et al. 2006). There are less than a dozen major research groups that investigate this unique phenotype, mainly in the U.S., Europe and Japan. Study populations have included isolated or ethnic groups of centenarians, mixed populations and populations from urban and rural areas. Selection of extreme phenotypes such as centenarians (and/or the oldest old) is likely to substantially increase power for detection of genes affecting longevity (Zhang and Risch 1996; Perls et al. 2002; Vijg and van Orsouw 2002; Perls and Terry 2003). A sub-group of oldest-old individuals with good cognitive and physical function who have not suffered from major age-related diseases may be even better candidates as they likely represent a “healthy aging phenotype”. Particularly valuable information about the heritability of the longevity trait may also be contributed by long-living persons who represent the top percentiles of long-lived birth cohorts (i.e. the 95th percentile and beyond) from their respective populations (Hadley and Rossi 2005).
Who represent the best controls?
The case-control design usually calls for cases and controls that differ only with regard to the phenotype under investigation. Ideally, all other variables, such as environmental factors, ethnicity, and gender should be as similar as possible in order to minimize confounding effects (Hirschhorn and Daly 2005). Long-term (preferably decades long) prospective cohort studies in which allelic variation in the long-lived group is compared with that of shorter lived controls are ideal but usually impractical, if not impossible. However, establishing “bio-banks” with stored samples will help to eliminate this problem in the future. At present, the most widely applied practical approach is one in which allele frequency in long-lived cases is compared with that of younger controls. However, a major problem associated with this type of study design is that we have no way of knowing how many of the controls will also live long.
Another methodological problem arises from the possibility that differences between the long-lived group and younger controls could be merely reflective of a change in population structure over time (Nebel et al. 2005). This may be easily brought about through recent population movements and/or admixture. However, there are some approaches that can help to compensate for these issues. These methods can be classified into two categories. Genomic control methods use the independent marker loci to adjust the distribution of a standard test statistic, while structured association methods infer the details of population structure to testing for association. Both methods may help compensate for some of the genetic differences between cases and controls that can arise from the lack of appropriate matching (Pritchard and Donnelly 2001).
The challenges of gene-environment interaction and other variables
Longevity associated allelic variation is context-dependent and the risk of mortality attributable to genotypes may depend on interactions with environmental risk factors that in turn vary by age, gender, ethnicity, geography, historical time period and/or bio-cultural context. As such, genes may have beneficial effects at younger ages but detrimental effects at older ages or the opposite pattern may hold, a phenomenon referred to as antagonistic pleiotropy (Toupance et al. 1998; Capri et al. 2006). Moreover, what may be good for men may not necessarily be beneficial for women. For example, some studies have found male or female-specific associations for certain polymorphisms and therefore stress the importance of gender as a major variable, suggesting that men and women may follow different trajectories to reach longevity (Lio et al. 2002).
Some environments may be more challenging to achieve exceptional longevity. De Benedictis and Franceschi (2006) recently touched upon this problem when they concluded that due to a more challenging environment, a better genetic make-up may be required to attain longevity in southern Europe than in northern Europe. Similarly, the centenarian of today is almost assuredly different from the centenarian of a generation ago. Moreover, what may be true for centenarians of today may not necessarily be true for their grandchildren. Since gene pools of populations change in response to environmental factors it is risky to assume that the genetic makeup of the current oldest-old generation is identical to that of past or future cohorts living in very different environments. The oldest-old still surviving today in most developed countries were born before demographic and epidemiological transitions sculpted a new bio-cultural landscape that has largely eliminated the mortality risks that were common at the turn of the century. Most younger cohorts have had the benefits of public health and modern life-saving medical technology, and most younger cohorts did not experience the world wars, socio-economic deprivation, or epidemics of deadly infectious disease that most of their forebears did. Thus, genetic variations that may have brought about the current generation of centenarians may not necessarily increase the life expectancy of a younger generation living in a vastly different bio-cultural /environmental milieu with a very different set of risk factors for morbidity and mortality.
Old genetic isolates may hold key to the new genetic research
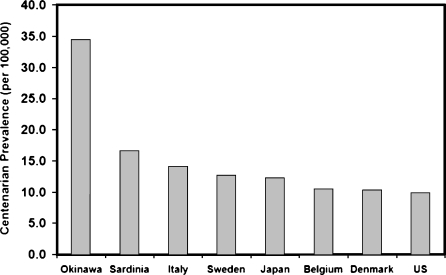
Of particular interest to the genetic epidemiology of exceptional longevity is that several isolated subpopulations appear to have longevity advantages. Iceland, Sardinia, and Okinawa, which exist within the Nordic countries, Italy, and Japan, respectively, have significantly lower old-age mortality, extended survival, and higher prevalence of long-lived individuals than surrounding areas (Gudmundsson et al. 2000; Poulain et al. 2004; Japan Health and Welfare Bureau for the Elderly 2006). The precise reasons are unclear but this may be due, in part, to clustering of advantageous genetic variants. This longevity advantage effect appears particularly strong in Okinawa, which has the highest prevalence of centenarians in Japan, if not the world at approximately 50 centenarians per 100,000 people in 2006 (Japan Health and Welfare Bureau for the Elderly 2006). Although we must be cautious when comparing centenarian ratios cross nationally due to methodological differences in calculating centenarian ratios and rapid year to year growth rates (Willcox et al., unpublished data), Figure 2 shows that prevalence of centenarians in Okinawa was substantially higher than other areas at 34/100,000 in the year 2002, in contrast to 16.5/100,000 in Sardinia, and approximately 10/100,000 in most Western countries (Japan Health and Welfare Bureau for the Elderly 2003; Poulain et al. 2004). This suggests a fertile location for exploring the genetics of human longevity.
Figure 2.
Centenarian ratios in selected regions in 2002. Note: Data are reported from respective government statistics for the year 2002 or the closest available calendar year
As mentioned previously, large movements of people and subsequent gene admixture (mixing of two or more genetically different populations) are substantial confounding factors in genetic studies. Genetic admixture can be seen in just one generation. These changes are of particular concern in countries with a large number of recent immigrants, such as the U.S. when using centenarians as a phenotype of longevity (Nebel et al. 2005). Even Caucasians in the U.S. are a markedly admixed population from diverse European origins. Therefore, the genetic composition of a U.S. centenarian sample is likely to differ substantially from that of a younger control population simply due to different migration patterns over different generations. Inappropriate matching of cases and controls under even modest levels of population stratification can cause both false-positive and false-negative findings. These problems do not exist in Okinawa for older generations, which have seen extremely limited immigration (Kerr 2000). Furthermore, population isolates are considered to be more useful in genome-wide association studies owing to increased linkage disequilibrium and decreased allelic diversity. This has recently been empirically demonstrated in a Pacific Island population where the long-range linkage disequilibrium around common alleles and limited diversity resulted in much improved efficiency in a genome wide association study, significantly augmenting the power to detect association of SNPs with particular complex phenotypes (Bonnen et al. 2006).
With the exception of our work on HLA genetic variants and two family studies there has been little study of the genetic determinants of longevity in this interesting population. However, a recent investigation of mitochondrial gene variants in Japan supports our past genetic work and argues strongly for further exploration of this population’s unique genotype (Tanaka et al. 2004). This study suggests that Okinawans may be a genetically distinct group that has several characteristics of a founder population, including less genetic diversity, and possess at least one mitochondrial haplotype (M7) linked to human longevity that has clustered in higher frequency than surrounding East Asian populations. Further work in this population would be of significant interest to the genetics of human longevity.
The future
Despite the observation that there is substantial genetic contribution to human longevity, little is known about what specific genetic effects are involved. As longevity is a very complex trait, several issues challenge our ability to identify its genetic influences, such as control for environmental confounders across time, the lack of precise phenotypes of aging and longevity, availability of appropriate study populations, statistical power, and study design. While recent advances in human genomics have made a comprehensive search for such genetic effects feasible, the aforementioned challenges still need to be met. There is a strong need for validation as well as replication of initial association findings in very large samples from a variety of different populations and sub-populations. Genetic background, age, gender, gene-environment interactions and other variables need sufficient consideration.
Moreover, the role of genes as modifiers of human lifespan requires rigorous testing through functional analyses before they can be regarded as genuine longevity genes. Recent studies have shown that the search for genes with moderate effects on complex traits at a whole-genome scale is now feasible and efforts in identifying specific genetic contributions to human longevity have begun to bear fruit. Given the appropriate study design, study populations and collective expertise we believe that significant advances will occur in the understanding of the genetics of human longevity in the coming years, the ultimate aim of which is to minimize disease and disability as we age.
References
- Akisaka M, Suzuki M. The bone density and activities of daily living in Okinawa centenarians. Hong Kong J Gerontol. 1996;10:453–457. [Google Scholar]
- Akisaka M, Suzuki M. Okinawa longevity study. Molecular genetic analysis of HLA genes in the very old. Nippon Ronen Igakkai Zasshi. 1998;35:294–298. doi: 10.3143/geriatrics.35.294. [DOI] [PubMed] [Google Scholar]
- Akisaka M, Asato L, Chan YC, Suzuki M, Uezato T, Yamamoto S. Energy and nutrient intakes of Okinawan centenarians. J Nutr Sci Vitaminol. 1996;42:241–248. doi: 10.3177/jnsv.42.241. [DOI] [PubMed] [Google Scholar]
- Akisaka M, Suzuki M, Inoko H. Molecular genetic studies on DNA polymorphism of the HLA class II genes associated with human longevity. Tissue Antigens. 1997;50:489–493. doi: 10.1111/j.1399-0039.1997.tb02904.x. [DOI] [PubMed] [Google Scholar]
- Allen M. Identity and resistance in Okinawa. Boston: Rowman and Littlefield; 2002. [Google Scholar]
- Altomare K, Greco V, Bellizzi D, Berardelli M, Dato S, DeRango F, et al. The allele (A)(-110) in the promoter region of the HSP70-1 gene is unfavorable to longevity in women. Biogerontology. 2003;4:215–220. doi: 10.1023/a:1025182615693. [DOI] [PubMed] [Google Scholar]
- Ambrosone CB, Freudenheim JL, Thompson PA, Bowman E, Vena JE, Marshall JR, et al. Manganese superoxide dismutase (MnSOD) genetic polymorphisms, dietary antioxidants, and risk of breast cancer. Cancer Res. 1999;59:602–606. [PubMed] [Google Scholar]
- Arai Y, Hirose N, Yamamura K, Nakazawa S, Shimizu K, Takayama M, et al. Deficiency of cholesteryl ester transfer protein and gene polymorphisms of lipoprotein lipase and hepatic lipase are not associated with longevity. J Mol Med. 2003;81:102–109. doi: 10.1007/s00109-002-0407-6. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Berman JR, Kenyon C. Healthy animals with extreme longevity. Science. 2003;302:611. doi: 10.1126/science.1089169. [DOI] [PubMed] [Google Scholar]
- Arking DE, Krebsova A, Macek M, Sr, Macek M, Jr, Arking A, Mian IS, et al. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA. 2002;99:856–861. doi: 10.1073/pnas.022484299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzmon G, Schechter C, Greiner W, Davidson D, Rennert G, Barzilai N. Clinical phenotype of families with longevity. J Am Geriatr Soc. 2004;52:274–277. doi: 10.1111/j.1532-5415.2004.52068.x. [DOI] [PubMed] [Google Scholar]
- Aviv A (2004) Telomeres and human aging: facts and fibs. Sci Aging Knowledge Environ 51:pe43 [DOI] [PubMed]
- Aviv A, Valdes AM, Spector TD (2006) Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol [Epub ahead of print on 22 Sept] [DOI] [PubMed]
- Barbieri M, Bonafe M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285:E1064–E1071. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- Bartke A, Chandrashekar V, Dominici F, Turyn D, Kinney B, Steger R. Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology. 2003;4:1–8. doi: 10.1023/a:1022448532248. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Atzmon G, Schechter C, Schaefer EJ, Cupples AL, Lipton R, et al. Unique lipoprotein phenotype and genotype associated with exceptional longevity. JAMA. 2003;290:2030–2040. doi: 10.1001/jama.290.15.2030. [DOI] [PubMed] [Google Scholar]
- Beekman M, Blauw GJ, Houwing-Duistermaat JJ, Brandt BW, Westendorp RG, Slagboom PE. Chromosome 4q25, microsomal transfer protein gene, and human longevity: novel data and a meta-analysis of association studies. J Gerontol Ser A Biol Sci Med Sci. 2006;61:355–362. doi: 10.1093/gerona/61.4.355. [DOI] [PubMed] [Google Scholar]
- Bekaert S, Pottelbergh I, Meyer T, Zmierczak H, Kaufman JM, Oostveldt P, et al. Telomere length versus hormonal and bone mineral status in healthy elderly men. Mech Ageing Dev. 2005;126:1115–1122. doi: 10.1016/j.mad.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Bekaert S, Meyer T, Oostveldt P. Telomere attrition as ageing biomarker. Anticancer Res. 2005;25:3011–3021. [PubMed] [Google Scholar]
- Bernstein AM, Willcox BJ, Tamaki H, Kunishima N, Suzuki M, Willcox DC, et al. First autopsy study of an Okinawan centenarian: absence of many age-related diseases. J Gerontol Ser A Biol Sci Med Sci. 2004;59:1195–1199. doi: 10.1093/gerona/59.11.1195. [DOI] [PubMed] [Google Scholar]
- Bischoff C, Graakjaer J, Petersen HC, Jeune B, Bohr VA, Koelvraa S, et al. No association between telomere length and survival among the elderly and oldest old. Epidemiology. 2006;17:190–194. doi: 10.1097/01.ede.0000199436.55248.10. [DOI] [PubMed] [Google Scholar]
- Bisoffi M, Heaphy CM, Griffith JK (2006) Telomeres: prognostic markers for solid tumors. Int J Cancer 119(10):2255–2260 [DOI] [PubMed]
- Bladbjerg EM, Andersen-Ranberg K, Maat MP, Kristensen SR, Jeune B, Gram J, et al. Longevity is independent of common variations in genes associated with cardiovascular risk. Thromb Haemost. 1999;82:1100–1105. [PubMed] [Google Scholar]
- Blanche H, Cabanne L, Sahbatou M, Thomas G. A study of French centenarians: are ACE and APOE associated with longevity? C R Acad Sci III. 2001;324:129–135. doi: 10.1016/s0764-4469(00)01274-9. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Bonafe M, Barbieri M, Marchegiani F, Olivieri F, Ragno E, et al. Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. J Clin Endocrinol Metab. 2003;88:3299–3304. doi: 10.1210/jc.2002-021810. [DOI] [PubMed] [Google Scholar]
- Bonnen PE, Pe’er I, Plenge RM, Salit J, Lowe JK, Shapero MH, et al. Evaluating potential for whole-genome studies in Kosrae, an isolated population in Micronesia. Nat Genet. 2006;38:214–217. doi: 10.1038/ng1712. [DOI] [PubMed] [Google Scholar]
- Braeckman BP, Vanfleteren JR (2006) Genetic control of longevity in C. elegans. Exp Gerontol [Epub ahead of print] [DOI] [PubMed]
- Broers JL, Ramaekers FC, Bonne G, Yaou RB, Hutchison CJ. Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev. 2006;86:967–1008. doi: 10.1152/physrev.00047.2005. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cancer and ageing: rival demons? Nature Rev. 2003;3:339–349. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]
- Candore G, Aquino A, Balistreri CR, Bulati M, Carlo D, Grimaldi MP, et al. Inflammation, longevity, and cardiovascular diseases: role of polymorphisms of TLR4. Ann NY Acad Sci. 2006;1067:282–287. doi: 10.1196/annals.1354.037. [DOI] [PubMed] [Google Scholar]
- Capri M, Salvioli S, Sevini F, Valensin S, Celani L, Monti D, et al. The genetics of human longevity. Ann NY Acad Sci. 2006;1067:252–263. doi: 10.1196/annals.1354.033. [DOI] [PubMed] [Google Scholar]
- Caruso C, Candore G, Colonna Romano G, Lio D, Bonafe M, Valensin S, et al. HLA, aging, and longevity: a critical reappraisal. Hum Immunol. 2000;61:942–949. doi: 10.1016/s0198-8859(00)00168-3. [DOI] [PubMed] [Google Scholar]
- Castro E, Ogburn CE, Hunt KE, Tilvis R, Louhija J, Penttinen R, et al. Polymorphisms at the Werner locus: I. Newly identified polymorphisms, ethnic variability of 1367Cys/Arg, and its stability in a population of Finnish centenarians. Am J Med Genet. 1999;82:399–403. [PubMed] [Google Scholar]
- Castro E, Edland SD, Lee L, Ogburn CE, Deeb SS, Brown G, et al. Polymorphisms at the Werner locus: II. 1074Leu/Phe, 1367Cys/Arg, longevity, and atherosclerosis. Am J Med Genet. 2000;95:374–380. [PubMed] [Google Scholar]
- Chan YC, Suzuki M, Yamamoto S. Dietary, anthropometric, hematological and biochemical assessment of the nutritional status of centenarians and elderly people in Okinawa, Japan. J Am Coll Nutr. 1997;16:229–235. doi: 10.1080/07315724.1997.10718679. [DOI] [PubMed] [Google Scholar]
- Chan YC, Suzuki M, Yamamoto S. Nutritional status of centenarians assessed by activity and anthropometric, hematological and biochemical characteristics. J Nutr Sci Vitaminol. 1997;43:73–81. doi: 10.3177/jnsv.43.73. [DOI] [PubMed] [Google Scholar]
- Chinnery PF, Samuels DC, Elson J, Turnbull DM. Accumulation of mitochondrial DNA mutations in ageing, cancer, and mitochondrial disease: is there a common mechanism? Lancet. 2002;360:1323–1325. doi: 10.1016/S0140-6736(02)11310-9. [DOI] [PubMed] [Google Scholar]
- Christensen K, McGue M, Yashin A, Iachine I, Holm NV, Vaupel JW. Genetic and environmental influences on functional abilities in Danish twins aged 75 years and older. J Gerontol Ser A Biol Sci Med Sci. 2000;55:M446–M452. doi: 10.1093/gerona/55.8.m446. [DOI] [PubMed] [Google Scholar]
- Christensen K, Gaist D, Vaupel JW, McGue M. Genetic contribution to rate of change in functional abilities among Danish twins aged 75 years or more. Am J Epidemiol. 2002;155:132–139. doi: 10.1093/aje/155.2.132. [DOI] [PubMed] [Google Scholar]
- Christensen K, Johnson TE, Vaupel JW. The quest for genetic determinants of human longevity: challenges and insights. Nat Rev Genet. 2006;7:436–448. doi: 10.1038/nrg1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Cockerham WC, Hattori H, Yamori Y. The social gradient in life expectancy: the contrary case of Okinawa in Japan. Soc Sci Med. 2000;51:115–122. doi: 10.1016/s0277-9536(99)00444-x. [DOI] [PubMed] [Google Scholar]
- Curb JD, Abbott RD, Rodriguez BL, Masaki K, Chen R, Sharp DS, et al. A prospective study of HDL-C and cholesteryl ester transfer protein gene mutations and the risk of coronary heart disease in the elderly. J Lipid Res. 2004;45:948–953. doi: 10.1194/jlr.M300520-JLR200. [DOI] [PubMed] [Google Scholar]
- Cutler RG. Evolution of human longevity and the genetic complexity governing aging rate. Proc Natl Acad Sci. 1975;72:4664–4668. doi: 10.1073/pnas.72.11.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dato S, Passarino G, Rose G, Altomare K, Bellizzi D, Mari V, et al. Association of the mitochondrial DNA haplogroup J with longevity is population specific. Eur J Hum Genet. 2004;12:1080–1082. doi: 10.1038/sj.ejhg.5201278. [DOI] [PubMed] [Google Scholar]
- Davis T, Kipling D. Werner syndrome as an example of inflamm-aging: possible therapeutic opportunities for a progeroid syndrome? Rejuvenation Res. 2006;9:402–407. doi: 10.1089/rej.2006.9.402. [DOI] [PubMed] [Google Scholar]
- De Benedictis G, Franceschi C (2006) The unusual genetics of human longevity. Sci Aging Knowledge Environ 2006:pe20 [DOI] [PubMed]
- Benedictis G, Carotenuto L, Carrieri G, Luca M, Falcone E, Rose G, et al. Gene/longevity association studies at four autosomal loci (REN, THO, PARP, SOD2) Eur J Hum Genet. 1998;6:534–541. doi: 10.1038/sj.ejhg.5200222. [DOI] [PubMed] [Google Scholar]
- Benedictis G, Rose G, Carrieri G, Luca M, Falcone E, Passarino G, et al. Mitochondrial DNA inherited variants are associated with successful aging and longevity in humans. FASEB J. 1999;13:1532–1536. doi: 10.1096/fasebj.13.12.1532. [DOI] [PubMed] [Google Scholar]
- Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579:2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- DeMichele A, Martin AM, Mick R, Gor P, Wray L, Klein-Cabral M, et al. Interleukin-6 -174G–>C polymorphism is associated with improved outcome in high-risk breast cancer. Cancer Res. 2003;63:8051–8056. [PubMed] [Google Scholar]
- Edo MD, Andres V. Aging, telomeres, and atherosclerosis. Cardiovasc Res. 2005;66:213–221. doi: 10.1016/j.cardiores.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Egan KM, Thompson PA, Titus-Ernstoff L, Moore JH, Ambrosone CB. MnSOD polymorphism and breast cancer in a population-based case-control study. Cancer Lett. 2003;199:27–33. doi: 10.1016/s0304-3835(03)00349-5. [DOI] [PubMed] [Google Scholar]
- Evert J, Lawler E, Bogan H, Perls T. Morbidity profiles of centenarians: survivors, delayers, and escapers. J Gerontol Ser A Biol Sci Med Sci. 2003;58:232–237. doi: 10.1093/gerona/58.3.m232. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Christensen K. The influence of genetic factors on physical functioning and exercise in second half of life. Scand J Med Sci Sports. 2003;13:9–18. doi: 10.1034/j.1600-0838.2003.20219.x. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, McGue M, Jeune B, Gaist D, Nybo H, Skytthe A, et al. Do children of long-lived parents age more successfully? Epidemiology. 2002;13:334–339. doi: 10.1097/00001648-200205000-00015. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Gaist D, Bathum L, Andersen K, McGue M, Vaupel JW, et al. Angiotensin I-converting enzyme (ACE) gene polymorphism in relation to physical performance, cognition and survival-a follow-up study of elderly Danish twins. Ann Epidemiol. 2003;13:57–65. doi: 10.1016/s1047-2797(02)00254-5. [DOI] [PubMed] [Google Scholar]
- Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63:168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- Gavrilov LA, Gavrilova NS. Evolutionary theories of aging and longevity. Scientific World Journal. 2002;2:339–356. doi: 10.1100/tsw.2002.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geesaman BJ, Benson E, Brewster SJ, Kunkel LM, Blanche H, Thomas G, et al. Haplotype-based identification of a microsomal transfer protein marker associated with the human lifespan. Proc Natl Acad Sci. 2003;100:14115–14120. doi: 10.1073/pnas.1936249100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Partridge L. Insulin/IGF signaling and ageing: seeing the bigger picture. Curr Opin Genet Dev. 2001;11:287–292. doi: 10.1016/s0959-437x(00)00192-1. [DOI] [PubMed] [Google Scholar]
- Gerdes LU, Jeune B, Ranberg KA, Nybo H, Vaupel JW. Estimation of apolipoprotein E genotype-specific relative mortality risks from the distribution of genotypes in centenarians and middle-aged men: apolipoprotein E gene is a “frailty gene,” not a “longevity gene”. Genet Epidemiol. 2000;19:202–210. doi: 10.1002/1098-2272(200010)19:3<202::AID-GEPI2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Greaves LC, Taylor RW. Mitochondrial DNA mutations in human disease. IUBMB Life. 2006;58:143–151. doi: 10.1080/15216540600686888. [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Gudmundsson H, Gudbjartsson DF, Frigge M, Gulcher JR, Stefansson K. Inheritance of human longevity in Iceland. Eur J Hum Genet. 2000;8:743–749. doi: 10.1038/sj.ejhg.5200527. [DOI] [PubMed] [Google Scholar]
- Gupta S, Agrawal A, Agrawal S, Su H, Gollapudi S. A paradox of immunodeficiency and inflammation in human aging: lessons learned from apoptosis. Immun Ageing. 2006;19:3–5. doi: 10.1186/1742-4933-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE epsilon 4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282:40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- Hadley EC, Rossi WK. Exceptional survival in human populations: National Institute on Aging perspectives and programs. Mech Ageing Dev. 2005;126:231–234. doi: 10.1016/j.mad.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Hasty P. The impact of DNA damage, genetic mutation and cellular responses on cancer prevention, longevity and aging: observations in humans and mice. Mech Ageing Dev. 2005;126:71–77. doi: 10.1016/j.mad.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Heijmans B, Westendorp R, Slagboom P. Common gene variants, mortality and extreme longevity in humans. Exp Gerontol. 2000;35:865–877. doi: 10.1016/s0531-5565(00)00171-6. [DOI] [PubMed] [Google Scholar]
- Hennekam RC (2006) Hutchinson-Gilford progeria syndrome: review of the phenotype. Am J Med Genet A [Epub ahead of print] [DOI] [PubMed]
- Hirai M, Suzuki S, Hinokio Y, Yamada T, Yoshizumi S, Suzuki C, et al. WRN gene 1367 Arg allele protects against development of type 2 diabetes mellitus. Diabetes Res Clin Pract. 2005;69:287–292. doi: 10.1016/j.diabres.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Hirose N, Arai Y, Yamamura K, Suzuki M, Akisaka M. Genetics of aging and longevity in Japanese centenarians. In: Tauchi H, Sato T, Watanabe T, editors. Medical research for the final stages of human aging. Aichi, Japan: Institute for Medical Science of Aging: Aichi Medical University Press; 1999. pp. 124–131. [Google Scholar]
- Hirschhorn JN, Daly MJ. Genome-wide association studies for common diseases and complex traits. Nat Rev Genet. 2005;6:95–108. doi: 10.1038/nrg1521. [DOI] [PubMed] [Google Scholar]
- Hjelmborg JV, Iachine I, Skytthe A, Vaupel JW, McGue M, Koskenvuo M, et al. Genetic influence on human lifespan and longevity. Hum Genet. 2006;119:312–321. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- Honig LS, Schupf N, Lee JH, Tang MX, Mayeux R. Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia. Ann Neurol. 2006;60:181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- Hung RJ, Boffetta P, Brennan P, Malaveille C, Gelatti U, Placidi D, et al. Genetic polymorphisms of MPO, COMT, MnSOD, NQO1, interactions with environmental exposures and bladder cancer risk. Carcinogenesis. 2004;25:973–978. doi: 10.1093/carcin/bgh080. [DOI] [PubMed] [Google Scholar]
- Hurme M, Lehtimaki T, Jylha M, Karhunen PJ, Hervonen A. Interleukin-6 -174G/C polymorphism and longevity: a follow-up study. Mech Ageing Dev. 2005;126:417–418. doi: 10.1016/j.mad.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Ishii N, Ishii T, Hartman PS (2006) The role of the electron transport gene SDHS on lifespan and cancer. Exp Gerontol 2006 [Epub ahead of print] [DOI] [PubMed]
- Japan Health and Welfare Bureau for the Elderly (2003) Japan annual centenarian report. Japan Health and Welfare Bureau for the Elderly, Tokyo, Japan
- Japan Health and Welfare Bureau for the Elderly (2006) Japan annual centenarian report. Japan Health and Welfare Bureau for the Elderly, Tokyo, Japan
- Jordan BD, Relkin NR, Ravdin LD, Jacobs AR, Bennett A, Gandy S. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA. 1997;278:136–140. [PubMed] [Google Scholar]
- Julien JP, Kriz J (2006) Transgenic mouse models of amyotrophic lateral sclerosis. Biochim Biophys Acta [Epub ahead of print] [DOI] [PubMed]
- Kagawa Y. Impact of Westernization on the nutrition of Japanese: changes in physique, cancer, longevity and centenarians. Prev Med. 1978;7:205–217. doi: 10.1016/0091-7435(78)90246-3. [DOI] [PubMed] [Google Scholar]
- Kerber RA, O’Brien E, Smith KR, Cawthon RM. Familial excess longevity in Utah genealogies. J Gerontol Ser A Biol Sci Med Sci. 2001;56:B130–B139. doi: 10.1093/gerona/56.3.b130. [DOI] [PubMed] [Google Scholar]
- Kerr G. Okinawa: the history of an island people. Boston: Tuttle; 2000. [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Kamei H, Aizu T, Arai Y, Takayama M, Nakazawa S, et al. Association analysis between longevity in the Japanese population and polymorphic variants of genes involved in insulin and insulin-like growth factor 1 signaling pathways. Exp Gerontol. 2004;39:1595–159. doi: 10.1016/j.exger.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Lebra WP. Okinawan religion: belief, ritual and social structure. Honolulu: University of Hawaii Press; 1986. [Google Scholar]
- Lee IM, Blair SN, Allison DB, Folsom AR, Harris TB, Manson JE, et al. Epidemiologic data on the relationships of caloric intake, energy balance, and weight gain over the life span with longevity and morbidity. J Gerontol Ser A Biol Sci Med Sci. 2001;56:7–19. doi: 10.1093/gerona/56.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- Lin P, Hsueh YM, Ko JL, Liang YF, Tsai KJ, Chen CY (2003) Analysis of NQO1, GSTP1, and MnSOD genetic polymorphisms on lung cancer risk in Taiwan. Lung Cancer 40:123-129 [DOI] [PubMed]
- Lin YJ, Seroude L, Benzer S. Extended life span and stress resistance in the Drosophila mutant Methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- Lio D, Scola L, Crivello A, Colonna-Romano G, Candore G, Bonafe M, et al. Gender-specific association between -1082 IL-10 promoter polymorphism and longevity. Genes Immun. 2002;3:30–33. doi: 10.1038/sj.gene.6363827. [DOI] [PubMed] [Google Scholar]
- Lithgow GJ, Walker GA. Stress resistance as a determinate of C. elegans lifespan. Mech Ageing Dev. 2002;123:765–771. doi: 10.1016/s0047-6374(01)00422-5. [DOI] [PubMed] [Google Scholar]
- Longo VD, Fabrizio P. Regulation of longevity and stress resistance: a molecular strategy conserved from yeast to humans? Cell Mol Life Sci. 2002;59:903–908. doi: 10.1007/s00018-002-8477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1345. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- Luft FC. Bad genes, good people, association, linkage, longevity and the prevention of cardiovascular disease. Clin Exp Pharmacol Physiol. 1999;26:576–579. doi: 10.1046/j.1440-1681.1999.03080.x. [DOI] [PubMed] [Google Scholar]
- Martin GM, Oshima J, Gray MD, Poot M. What geriatricians should know about the Werner syndrome. J Am Geriatr Soc. 1999;47:1136–1144. doi: 10.1111/j.1532-5415.1999.tb05240.x. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz CM, Gussekloo J, Heemst D, Zglinicki T, Westendorp RG. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4:287–290. doi: 10.1111/j.1474-9726.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- Masoro EJ (2006) Dietary restriction-induced life extension: a broadly based biological phenomenon. Biogerontology [Epub ahead of print] [DOI] [PubMed]
- McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, et al. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276:1560–1563. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- Merriwether DA, Clark AG, Ballinger SW, Schurr TG, Soodyall H, Jenkins T, et al. The structure of human mitochondrial DNA variation. J Mol Evol. 1991;33:543–555. doi: 10.1007/BF02102807. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Hoeijmakers JH, Niedernhofer LJ. Divide and conquer: nucleotide excision repair battles cancer and ageing. Curr Opin Cell Biol. 2003;15:232–240. doi: 10.1016/s0955-0674(03)00018-8. [DOI] [PubMed] [Google Scholar]
- Mitrunen K, Sillanpaa P, Kataja V, Eskelinen M, Kosma VM, Benhamou S, et al. Association between manganese superoxide dismutase (MnSOD) gene polymorphism and breast cancer risk. Carcinogenesis. 2001;22:827–829. doi: 10.1093/carcin/22.5.827. [DOI] [PubMed] [Google Scholar]
- Nebel A, Schreiber S (2005) Allelic variation and human longevity. Sci Aging Knowledge Environ 2005:pe23 [DOI] [PubMed]
- Nebel A, Croucher PJP, Stiegeler R, Nikolaus S, Krawczak M, Schreiber S. No association between microsomal triglyceride transfer protein (MTP) haplotype and longevity in humans. Proc Natl Acad Sci. 2005;102:7906–7909. doi: 10.1073/pnas.0408670102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi AK, Hervonen A, Hurme M, Karhunen PJ, Jylha M, Majamaa K. Mitochondrial DNA polymorphisms associated with longevity in a Finnish population. Hum Genet. 2003;112:29–33. doi: 10.1007/s00439-002-0843-y. [DOI] [PubMed] [Google Scholar]
- Niemi AK, Moilanen JS, Tanaka M, Hervonen A, Hurme M, Lehtimaki T, et al. A combination of three common inherited mitochondrial DNA polymorphisms promotes longevity in Finnish and Japanese subjects. Eur J Hum Genet. 2005;13:166–170. doi: 10.1038/sj.ejhg.5201308. [DOI] [PubMed] [Google Scholar]
- Ogura C, Nakamoto H, Uema T, Yamamoto K, Yonemori T, Yoshimura T. Prevalence of senile dementia in Okinawa, Japan. Int J Epidemiol. 1995;24:373–380. doi: 10.1093/ije/24.2.373. [DOI] [PubMed] [Google Scholar]
- Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, et al. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol Aging. 2003;24:77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Peila R, Launer LJ. Inflammation and dementia: epidemiologic evidence. Acta Neurol Scand. 2006;185:102–106. doi: 10.1111/j.1600-0404.2006.00693.x. [DOI] [PubMed] [Google Scholar]
- Perls T, Terry D. Genetics of exceptional longevity. Exp Gerontol. 2003;38:725–730. doi: 10.1016/s0531-5565(03)00098-6. [DOI] [PubMed] [Google Scholar]
- Perls TT, Bubrick E, Wager CG, Vijg J, Kruglyak L. Siblings of centenarians live longer. Lancet. 1998;351:1560. doi: 10.1016/S0140-6736(05)61126-9. [DOI] [PubMed] [Google Scholar]
- Perls T, Kunkel LM, Puca AA. The genetics of exceptional human longevity. JAGS. 2002;50:339–368. doi: 10.1046/j.1532-5415.2002.49283.x. [DOI] [PubMed] [Google Scholar]
- Pes GM, Lio D, Carru C, Deiana L, Baggio G, Franceschi C, et al. Association between longevity and cytokine gene polymorphisms. A study in Sardinian centenarians. Aging Clin Exp Res. 2004;16:244–248. doi: 10.1007/BF03327391. [DOI] [PubMed] [Google Scholar]
- Pollex RL, Hegele RA. Hutchinson-Gilford progeria syndrome. Clin Genet. 2004;66:375–381. doi: 10.1111/j.1399-0004.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- Poulain M, Pes GM, Grasland C, Carru C, Ferrucci L, Baggio G, et al. Identification of a geographic area characterized by extreme longevity in the Sardinia Island: the AKEA study. Exp Gerontol. 2004;39:1423–1429. doi: 10.1016/j.exger.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Donnelly P. Case-control studies of association in structured or admixed populations. Theor Popul Biol. 2001;60:227–237. doi: 10.1006/tpbi.2001.1543. [DOI] [PubMed] [Google Scholar]
- Puca AA, Daly MJ, Brewster SJ, Matise TC, Barrett J, Shea-Drinkwater M, et al. A genome-wide scan for linkage to human exceptional longevity identifies a locus on chromosome 4. Proc Natl Acad Sci. 2001;98:10505–10508. doi: 10.1073/pnas.181337598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea IM, Ross OA, Armstrong M, McNerlan S, Alexander DH, Curran MD, et al. Interleukin-6-gene C/G 174 polymorphism in nonagenarian and octogenarian subjects in the BELFAST study. Reciprocal effects on IL-6, soluble IL-6 receptor and for IL-10 in serum and monocyte supernatants. Mech Ageing Dev. 2003;124:555–561. doi: 10.1016/s0047-6374(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Rea IM, McKeown PP, McMaster D, Young IS, Patterson C, Savage MJ, et al. Paraoxonase polymorphisms PON1 192 and 55 and longevity in Italian centenarians and Irish nonagenarians. A pooled analysis. Exp Gerontol. 2004;39:629–635. doi: 10.1016/j.exger.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Rivadeneira F, Houwing-Duistermaat JJ, Vaessen N, Vergeer-Drop JM, Hofman A, Pols HA, et al. Association between an insulin-like growth factor I gene promoter polymorphism and bone mineral density in the elderly: the Rotterdam Study. J Clin Endocrinol Metab. 2003;88:3878–3884. doi: 10.1210/jc.2002-021813. [DOI] [PubMed] [Google Scholar]
- Rodriguez BL, Lau N, Burchfiel CM, Abbott RD, Sharp DS, Yano K, et al. Glucose intolerance and 23-year risk of coronary heart disease and total mortality: the Honolulu Heart Program. Diabetes Care. 1999;22:1262–1265. doi: 10.2337/diacare.22.8.1262. [DOI] [PubMed] [Google Scholar]
- Rogina B, Reenan RA, Nilsen SP, Helfand SL. Extended life-span conferred by co transporter gene mutations in Drosophila. Science. 2000;290:2137–2140. doi: 10.1126/science.290.5499.2137. [DOI] [PubMed] [Google Scholar]
- Rose G, Dato S, Altomare K, Bellizzi D, Garasto S, Greco V, et al. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol. 2003;38:1065–1070. doi: 10.1016/s0531-5565(03)00209-2. [DOI] [PubMed] [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, et al. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- Sampayo JN, Gill MS, Lithgow GJ. Oxidative stress and aging-the use of superoxide dismutase/catalase mimetics to extend lifespan. Biochem Soc Trans. 2003;31:1305–1307. doi: 10.1042/BST0311305. [DOI] [PubMed] [Google Scholar]
- Sanabe E, Ashitomi I, Suzuki M. Social and medical survey of centenarians. Okinawa J Public Health. 1977;9:98–106. [Google Scholar]
- Schachter F. Causes, effects, and constraints in the genetics of human longevity. Am J Hum Genet. 1998;62:1008–1014. doi: 10.1086/301849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter F, Faure-Delanef L, Guenot F, Rouger H, Froguel P, Lesueur-Ginot L, et al. Genetic associations with human longevity at the APOE and ACE loci. Nat Genet. 1994;6:29–32. doi: 10.1038/ng0194-29. [DOI] [PubMed] [Google Scholar]
- Scola L, Lio D, Crivello A, Candore G, Forte GI, Colonna-Romano G, et al. Analysis of HLA-DQA, HLA-DQB frequencies in a group of Sardinian centenarians. Rejuvenation Res. 2006;9:157–160. doi: 10.1089/rej.2006.9.157. [DOI] [PubMed] [Google Scholar]
- Service S, Molina J, Deyoung J, Jawaheer D, Aldana I, Vu T, et al. Magnitude and distribution of linkage disequilibrium in population isolates and implications for genome-wide association studies. Nat Genet. 2006;38:556–560. doi: 10.1038/ng1770. [DOI] [PubMed] [Google Scholar]
- Sho H. History and characteristics of Okinawan longevity food. Asia Pac J Clin Nutr. 2001;10:159–164. doi: 10.1111/j.1440-6047.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Singh R, Kolvraa S, Bross P, Gregersen N, Andersen Nexo B, et al. Association between low self-rated health and heterozygosity for –110A > C polymorphism in the promoter region of HSP70-1 in aged Danish twins. Biogerontology. 2004;5:169–176. doi: 10.1023/B:BGEN.0000031154.57176.4f. [DOI] [PubMed] [Google Scholar]
- Stoehlmacher J, Ingles SA, Park DJ, Zhang W, Lenz HJ. The -9Ala/-9Val polymorphism in the mitochondrial targeting sequence of the manganese superoxide dismutase gene (MnSOD) is associated with age among Hispanics with colorectal carcinoma. Oncol Rep. 2002;9:235–238. [PubMed] [Google Scholar]
- Suzuki M, Hirose N. Endocrine function of centenarians. In: Tauchi H, Sato T, Watanabe T, editors. Medical research for the final stages of human aging. Aichi, Japan: Institute for Medical Science of Aging: Aichi Medical University Press; 1999. pp. 101–110. [Google Scholar]
- Suzuki M, Mori H, Asato T, Sakugawa H, Ishii T, Hosoda Y. A medical study of centenarians: case control study of genetic factors, family history and longevity. Japan J Geriatr. 1985;22:457–467. doi: 10.3143/geriatrics.22.457. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Willcox BJ, Willcox DC. Implications from and for food cultures for cardiovascular disease: longevity. Asia Pac J Clin Nutr. 2001;10:165–171. doi: 10.1111/j.1440-6047.2001.00219.x. [DOI] [PubMed] [Google Scholar]
- Takata H, Suzuki M, Ishii T, Sekiguchi S, Iri H. Influence of major histocompatibility complex region genes on human longevity among Okinawan-Japanese centenarians and nonagenarians. Lancet. 1987;10:824–826. doi: 10.1016/s0140-6736(87)91015-4. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Gong J, Zhang J, Yamada Y, Borgeld HJ, Yagi K. Mitochondrial genotype associated with longevity and its inhibitory effect on mutagenesis. Mech Ageing Dev. 2000;116:65–76. doi: 10.1016/s0047-6374(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Cabrera VM, Gonzalez AM, Larruga JM, Takeyasu T, Fuku N, et al. Mitochondrial genome variation in eastern Asia and the peopling of Japan. Genome Res. 2004;14:1832–1850. doi: 10.1101/gr.2286304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Taufer M, Peres A, Andrade VM, Oliveira G, Sa G, Canto ME, et al. Is the Val16Ala manganese superoxide dismutase polymorphism associated with the aging process? J Gerontol Ser A Biol Sci Med Sci. 2005;60:432–438. doi: 10.1093/gerona/60.4.432. [DOI] [PubMed] [Google Scholar]
- Todesco L, Angst C, Litynski P, Loehrer F, Fowler B, Haefeli WE. Methylenetetrahydrofolate reductase polymorphism, plasma homocysteine and age. Eur J Clin Investig. 1999;29:1003–1009. doi: 10.1046/j.1365-2362.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- Todoriki H, Willcox DC, Willcox BJ. The effects of post-war dietary change on longevity and health in Okinawa. Okinawa J Amer Studies. 2004;1:52–61. [Google Scholar]
- Toupance B, Godelle B, Gouyon PH, Schachter F. A model for antagonistic pleiotropic gene action for mortality and advanced age. Am J Hum Genet. 1998;62:1525–1534. doi: 10.1086/301865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BJ, Atkin JD, Farg MA, Zang da W, Rembach A, Lopes EC, et al. Impaired extracellular secretion of mutant superoxide dismutase 1 associates with neurotoxicity in familial amyotrophic lateral sclerosis. J Neurosci. 2005;25:108–117. doi: 10.1523/JNEUROSCI.4253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaessen N, Heutink P, Janssen JA, Witteman JC, Testers L, Hofman A, et al. A polymorphism in the gene for IGF-I: functional properties and risk for type 2 diabetes and myocardial infarction. Diabetes. 2001;50:637–642. doi: 10.2337/diabetes.50.3.637. [DOI] [PubMed] [Google Scholar]
- Vaillent GE. The association of ancestral longevity with successful aging. J Gerontol. 1991;46:P292–P298. doi: 10.1093/geronj/46.6.p292. [DOI] [PubMed] [Google Scholar]
- Heemst D, Beekman M, Mooijaart SP, Heijmans BT, Brandt BW, Zwaan BJ, et al. Reduced insulin/IGF-1 signalling and human longevity. Aging Cell. 2005;4:79–85. doi: 10.1111/j.1474-9728.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- Vaupel JW. The remarkable improvements in survival at older ages. Philos Trans R Soc Lond B Biol Sci. 1997;352:1799–1804. doi: 10.1098/rstb.1997.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijg J, Orsouw N. Searching for determinants of human aging and longevity: opportunities and challenges. Mech Ageing Dev. 2002;123:195–205. doi: 10.1016/s0047-6374(01)00346-3. [DOI] [PubMed] [Google Scholar]
- Wang XY, Hurme M, Jylha M, Hervonen A. Lack of association between human longevity and polymorphisms of IL-1 cluster, IL-6, IL-10 and TNF-alpha genes in Finnish nonagenarians. Mech Ageing Dev. 2001;123:29–38. doi: 10.1016/s0047-6374(01)00338-4. [DOI] [PubMed] [Google Scholar]
- Warner HR (2003) Subfield history: use of model organisms in the search for human aging genes. Sci Aging Knowledge Environ 2003(6):RE1 [DOI] [PubMed]
- Warner HR. Longevity genes: from primitive organisms to humans. Mech Ageing Dev. 2005;126:235–242. doi: 10.1016/j.mad.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Willcox DC. Okinawan longevity: where do we go from here? Nutr Dietetics. 2005;8:9–17. [Google Scholar]
- Willcox BJ, Willcox DC, Suzuki M. The Okinawa diet. New York: Random House; 2004. [Google Scholar]
- Willcox BJ, Willcox DC, He Q, Curb JD, Suzuki M. Siblings of Okinawan centenarians share lifelong mortality advantages. J Gerontol Ser A Biol Sci Med Sci. 2006;61:345–354. doi: 10.1093/gerona/61.4.345. [DOI] [PubMed] [Google Scholar]
- Willcox DC, Willcox BJ, Todoriki H, Curb JD, Suzuki M. Caloric restriction and human longevity: what can we learn from the Okinawans? Biogerontology. 2006;7:173–177. doi: 10.1007/s10522-006-9008-z. [DOI] [PubMed] [Google Scholar]
- Woodson K, Tangrea JA, Lehman TA, Modali R, Taylor KM, Snyder K, et al. Manganese superoxide dismutase (MnSOD) polymorphism, alpha-tocopherol supplementation and prostate cancer risk in the alpha-tocopherol, beta-carotene cancer prevention study (Finland) Cancer Causes Control. 2003;214:513–518. doi: 10.1023/a:1024840823328. [DOI] [PubMed] [Google Scholar]
- Ye L, Miki T, Nakura J, Oshima J, Kamino K, Rakugi H, et al. Association of a polymorphic variant of the Werner helicase gene with myocardial infarction in a Japanese population. Am J Med Genet. 1997;68:494–498. doi: 10.1002/(sici)1096-8628(19970211)68:4<494::aid-ajmg30>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Zhang H, Risch N. Mapping quantitative-trait loci in humans by use of extreme concordant sib pairs: selected sampling by parental phenotypes. Am J Hum Genet. 1996;59:951–957. [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Asin-Cayuela J, Fish J, Michikawa Y, Bonafe M, Olivieri F, et al. Strikingly higher frequency in centenarians and twins of mtDNA mutation causing remodeling of replication origin in leukocytes. Proc Natl Acad Sci. 2003;100:1116–1121. doi: 10.1073/pnas.242719399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubenko GS, Stiffler JS, Hughes HB, Fatigati III, Zubenko MJ. Genome survey for loci that influence successful aging: sample characterization, method validation, and initial results for the Y chromosome. Am J Geriatr Psychiatry. 2002;10:619–630. [PubMed] [Google Scholar]