Abstract
Many tumors have been reported to coexist with cerebral aneurysm. However, intracranial dermoid cysts associated with cerebral aneurysm are very rare. We report a case in which rupture of a cerebral aneurysm resulted in a ruptured dermoid cyst. We present this interesting case and review current literature about the relationship between tumors and aneurysm formation.
Keywords: Dermoid cyst, Aneurysm, Rupture
INTRODUCTION
Intracranial dermoid cyst is a rare brain tumor that is a developmental malformation originating from ectopic cell arrest that causes a secondary defect in neural tube formation. Brain tumors associated with cerebral aneurysm have been reported occasionally, however, there is only one reported case of delayed formation of cerebral aneurysm after rupture of a dermoid cyst1,7,14). Furthermore, although many cases of spontaneous rupture of an intracranial dermoid cyst have been described, there are no reports of association with ruptured cerebral aneurysm2,6,10,18). We present an interesting case of coexistence of intracranial dermoid cyst and cerebral aneurysm that resulted in a ruptured dermoid cyst.
CASE REPORT
A 50-year-old man was admitted to the hospital with a stuporous mental state after the sudden onset of a severe headache. A focal neurologic deficit was not found and the brain stem reflex was intact. The patient did not have a previous history of headache or other symptoms and signs before admission. There was no history of hypertension or diabetes mellitus.
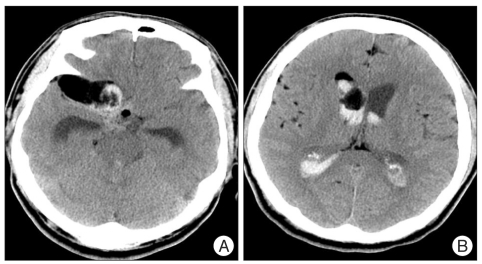
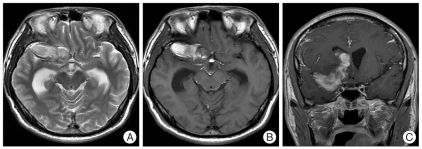
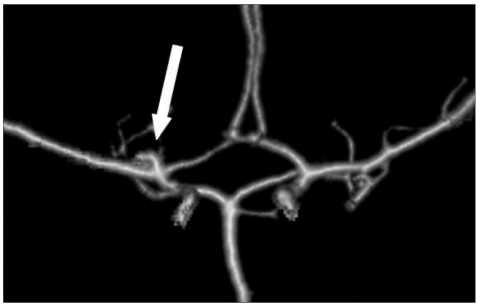
A computed tomographic (CT) scan revealed a subarachnoid hemorrhage in the basal cistern and a hematoma in both lateral ventricles (Fig. 1A). Many dots of low density that were suspected fat droplets were scattered in the basal cistern and subarachnoid space of both hemispheres (Fig. 1B). A 43×21 mm sized, oval shaped, and very low density mass was evident in the right temporal pole and frontal base. The mass compressed the frontal and temporal lobe and extended to the anterior horn of the right lateral ventricle through the frontal lobe. The size of the lateral ventricle was slightly increased. Magnetic resonance imaging (MRI) showed mainly high signal intensity in T2 and T1 weighted image (WI) without enhancement effect with gadolinium and no high signal intensity in T2WI surrounding the mass, which was thought to be cerebral edema (Fig. 2). The mass did not show a restricted pattern in diffusion WI. Scattered spots of high signal intensity on T1WI were found in the cerebral sulci of both hemispheres. On 3-dimensional cerebral angiography, a small, saccular, and superiorly directional aneurysm with a daughter sac was interpreted in the bifurcation of the right internal carotid artery (Fig. 3). No other vascular abnormality such as stenosis or vasospasm was observed.
Fig. 1.
Computed tomographic scan showing a subarachnoid hemorrhage in basal cistern and a mass in the right temporal pole and frontal base (A). hematoma in both lateral ventricles and scattered fat droplet in subarachnoid space of both hemispheres (B).
Fig. 2.
High signal and oval mass to compress frontal and temporal lobe reveals in T2 (A) and T1WI (B), extended mass without enhancement to the anterior horn of right lateral ventricle through the frontal lobe shows on gadolinium enhanced T1WI (C). WI : weighted image.
Fig. 3.
Three-dimensional cerebral angiography demonstrating a small, saccular, and superiorly directional aneurysm with a daughter sac in bifurcation of right internal carotid artery.
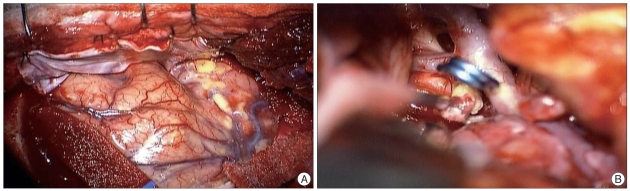
Emergency removal of the mass and clipping of the aneurysm were performed via the trans-sylvian approach by pterional craniotomy. When the dura mater was opened, many fat droplets floated in the subarachnoid space of the hemisphere (Fig. 4A). After dissection and opening of the sylvian fissure, internal decompression of the mass was performed followed by dissection of the arachnoid membrane from the tumor capsule and adjacent cerebral vessel. The mass was very soft and yellowish on the microscopic field. Subcutaneous appendages such as hair, keratinous material, and fat were present in the mass contents. After partial resection of the mass and complete dissection of the tumor capsule, strong adhesion between the tumor capsule and aneurysm wall could be seen; however, the aneurysm was not encased by the tumor capsule. After carefully resolving the adhesion between the mass capsule and aneurismal wall, we could safely clip the aneurysm neck (Fig. 4B). The main mass was removed en bloc, and then the remnant and extended mass in the right lateral ventricle was pulled totally through the frontal base opening created by rupture of the tumor. The final pathological diagnosis was confirmed as typical dermoid cyst (Fig. 5).
Fig. 4.
Intraoperative photographs. A : Many floated fat droplets in the subarachnoid space of temporal lobe and sylvian fissure. B : Complete obliteration of aneurysm in bifurcation of internal carotid artery was performed by clipping after removal of main mass on basal portion.
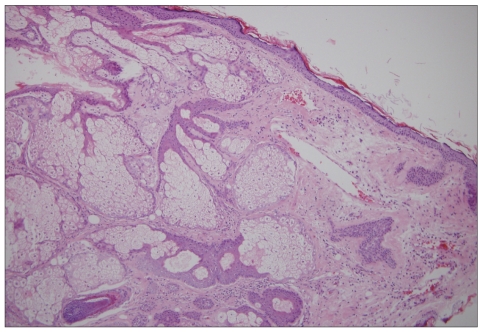
Fig. 5.
The cyst consists of keratinized squamous epithelium, hair follicles and abundant sebaceous glands (H&E, ×100).
Postoperatively, the patient gradually recovered his mentality to a drowsy state after 4 days and a focal neurologic deficit was not found. On the 7th day after the operation, the patient experienced a febrile sensation and severe neck stiffness. The CSF contained 200 WBC count, 284 mg/dL protein, and 37 mg/dL glucose. No growth of pathogen was detected in CSF and serum culture. The patient's symptoms and signs were improved with only steroid administration. Conventional angiography on the 29th postoperative day revealed the secured aneurysm and no other vascular lesion. Three months after the operation there was no recurrence of the cyst on CT scan despite the existence of fat droplets in the subarachoid space of hemisphere.
DISCUSSION
Intracranial dermoid cysts are rare benign neoplasms that are usually located midline and are caused by embryological malformation during development of the neural tube between the third and fifth weeks. The cysts are lined by squamous epithelium and contain skin appendages such as hair follicles, sebaceous glands, nails, and teeth. The mass enlarges as a result of its increased content of glandular secretions and epithelial desquamation, and as the mass grows many symptoms develop due to the compressed neural structure. On CT scan, dermoid cysts occasionally show fluid content and very low density due to lipid materials. Marked high signal intensity of T1WI without enhancement and iso- or hyperintensity on T2WI is usually demonstrated on MRI2,6,8,10,18). The lesion may have a calcification area. The differential diagnoses to consider on radiological findings are craniopharyngioma, epidermoid cyst, arachnoid cyst, teratoma, and lipoma. The treatment of choice is total surgical resection with careful dissection between the cyst capsule and neurovascular structure.
Spontaneous rupture of dermoid cysts develops occasionally as a result of tumor growth caused by increased content. However, rupture of the cyst due to external factors such as a closed head injury has been reported and can also occur as a result of vascular lesions, similar to our case13). The clinical manifestations of a ruptured dermoid cyst are sudden headache, seizure, embolic events, visual disturbance, and rarely no symptoms. On image study, the diagnosis of rupture is typically confirmed by dot-like lesions in the subarachnoid space5,19). Rupture of the cyst can also lead to fatal chemical meningitis9). Treatment of a ruptured dermoid cyst involves surgical removal of the main mass and scattered fat droplets in the subarachnoid space, and administration of intravenous steroids. At the 7th postoperative day, our patient had signs of meningeal irritation and febrile sense. The CSF profiles indicated chemical meningitis by the elevated WBC count and protein, and decreased glucose. There was no growth of pathogen in CSF and serum culture, and other laboratory studies were within normal range except for mild leukocytosis. The patient recovered from chemical meningitis with only steroid administration.
In previously described cases of ruptured dermoid cyst, cerebral vasospasm was reported occasionally and many probable pathogeneses of this phenomenon were discussed3,11,16). When a dermoid cyst is ruptured, fat droplets from the cyst contents spread throughout the subarachnoid space and ventricle. Direct irritation of the cerebral vessel wall by these lipid substances could result in vasospasm. Increased intracranial pressure or mechanical irritation of the cyst wall by the vessel wall was also discussed. Fortunately, our patient had no ischemic symptoms and signs, and was shown to lack silent cerebral vasospasm by angiography at pre- and postoperative periods.
Many cases of brain tumor coexisting with cerebral aneurysm have been reported previously4,7,14,15). A review of the literature showed that the incidence of presentation with brain tumor and cerebral aneurysm was approximately 0.2% to 1.1%. The highest incidence was found in meningioma, glioma, and pituitary adenoma, and the most commonly involved cerebral vessel was the internal carotid artery14). This study was designed to compare two groups of tumor, convexity and basal tumor. In the convexity tumor group, the most commonly involved cerebral vessel was the middle cerebral artery, followed by the anterior cerebral artery. In contrast, the basal tumors were more likely to be associated with the internal carotid artery than other tumor locations. In our case, the dermoid cyst was located in the basal portion and the aneurysm was found on the internal carotid artery, similar to the previous study. Considering the locations of the tumor and cerebral aneurysm, we could speculate that a possible factor influencing aneurysm formation was a direct effect of the tumor on the adjacent cerebral artery.
Although there is only one reported case of a dermoid cyst associated with unruptured cerebral aneurysm, many cases of other intracranial tumors have been reported and discussed with respect to the possible pathogenesis of aneurysm formation4,12,14,15). A previous study of a dermoid cyst by Ahmad et al.1 reported spontaneous rupture that was not caused by a ruptured cerebral aneurysm. Although the cerebral aneurysm was not diagnosed at the time of rupture of the cyst, the saccular aneurysm was found during surgery for removal of the dermoid cyst after 6 years. This report proposed strong adhesion resulting from the inflammatory response of the tumor capsule as a possible pathogenesis. For other tumors, many hypothesis have been proposed : 1) mechanical effect of increased blood flow due to a higher blood supply to tumors such as meningioma and glioma, 2) atherosclerotic and generative changes caused by direct hormonal effects of acromegaly and indirect hormonal effects of hypertension and diabetes mellitus, 3) direct and actual metastasis of the tumor to the blood vessel wall4,12,15,17,20). However, the exact pathogenesis of aneurysm formation related to tumor remains still obscure. Because the dermoid cyst was an avascular tumor with a benign nature pathologically and our case was a same finding, pathogenesis methods involving high blood flow and metastasis to vessel wall are not relevant. In the operative field, the cyst and cerebral aneurysm showed strong adhesion, therefore direct mechanical effects of the capsule wall on the cerebral vessel wall might have contributed to development of the aneursym.
The initial symptoms at presentation of patients with tumor and aneurysm are important for diagnosis and management. From a review of the literature, most patients with coexistence of tumor and aneurysm have symptoms caused by the tumor; only 22% of patients complained of subarachnoid hemorrhage due to aneurysm rupture14). For patients with symptoms of the tumor, cerebral aneurysm can be missed without further evaluation such as angiography, especially in basal tumors. As our patient was admitted with symptoms of a ruptured aneurysm, we could treat the two different diseases in an informed way before the operation. Otherwise, we would incur the risk of a ruptured cerebral aneurysm during removal of the tumor.
CONCLUSION
Among intracranial tumors, the dermoid cyst is an uncommon benign neoplasm, and its association with cerebral aneurysm is very rare. We experienced a case in which rupture of cerebral aneurysm resulted in a ruptured intracranial dermoid cyst. Although many hypotheses have been proposed for other tumors, the relationship between the mass and aneurysm formation is unclear. Considering the incidence and probable pathogenesis, it is likely that the brain tumor and adjacent cerebral aneurysm have a close relationship, especially direct mechanical effects of the capsule wall on the cerebral vessel wall in our case. Therefore, it is important to consider the possibility of an associated vascular lesion, and evaluation of the cerebral artery is needed in brain tumors of the basal portion.
References
- 1.Ahmad I, Tominaga T, Ogawa A, Yoshimoto T. Ruptured suprasellar dermoid associated with middle cerebral artery aneurysm : case report. Surg Neurol. 1992;38:341–346. doi: 10.1016/0090-3019(92)90019-j. [DOI] [PubMed] [Google Scholar]
- 2.el Quessar A, Chakir N, Bouyaakoub F, el Hassani MR, Jiddane M, Boukhrissi N. [Spontaneous rupture of an intracerebral dermoid cyst] Ann Radiol (Paris) 1996;39:253–256. [PubMed] [Google Scholar]
- 3.Ford K, Drayer B, Osborne D, Dubois P. Case report. Transient cerebral ischemia as a manifestation of ruptured intracranial dermoid cyst. J Comput Assist Tomogr. 1981;5:895–897. doi: 10.1097/00004728-198112000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Goodman ML, Nelson PB. Association of an epidermoid tumor with an aneurysm of the anterior communicating artery. Neurosurgery. 1988;23:392–395. doi: 10.1227/00006123-198809000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Hahn FJ, Ong E, McComb RD, Mawk JR, Leibrock LG. MR imaging of ruptured intracranial dermoid. J Comput Assist Tomogr. 1986;10:888–889. doi: 10.1097/00004728-198609000-00040. [DOI] [PubMed] [Google Scholar]
- 6.Heinze A, Saßen R, Hoeschel M. Ruptured intracranial dermoid cyst. Clin Neuroradiol. 2008;18:127–128. [Google Scholar]
- 7.Jakubowski J, Kendall B. Coincidental aneurysms with tumours of pituitary origin. J Neurol Neurosurg Psychiatry. 1978;41:972–979. doi: 10.1136/jnnp.41.11.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kay CS, Kim G, Chee SH, Park BW. A case of large dermoid cyst. J Korean Neurosurg Soc. 1977;6:549–554. [Google Scholar]
- 9.Lunardi P, Missori P, Rizzo A, Gagliardi FM. Chemical meningitis in ruptured intracranial dermoid. Case report and review of the literature. Surg Neurol. 1989;32:449–452. doi: 10.1016/0090-3019(89)90010-4. [DOI] [PubMed] [Google Scholar]
- 10.Manlapaz JS. Ruptured intracranial dermoid; report of a case and survey of previously reported cases. Am J Surg. 1960;100:723–730. doi: 10.1016/0002-9610(60)90415-3. [DOI] [PubMed] [Google Scholar]
- 11.Mikhael MA. Transient spasm of carotid siphon complicating ruptured cranial dermoid cyst. Radiology. 1982;144:824. doi: 10.1148/radiology.144.4.7111731. [DOI] [PubMed] [Google Scholar]
- 12.Momma F, Beck H, Miyamoto T, Nagao S. Intracranial aneurysm due to metastatic choriocarcinoma. Surg Neurol. 1986;25:74–76. doi: 10.1016/0090-3019(86)90119-9. [DOI] [PubMed] [Google Scholar]
- 13.Phillips WE, 2nd, Martinez CR, Cahill DW. Ruptured intracranial dermoid tumor secondary to closed head trauma. Computed tomography and magnetic resonance imaging. J Neuroimaging. 1994;4:169–170. doi: 10.1111/jon199443169. [DOI] [PubMed] [Google Scholar]
- 14.Pia HW, Obrador S, Martin JG. Association of brain tumours and arterial intracranial aneurysms. Acta Neurochir (Wien) 1972;27:189–204. doi: 10.1007/BF01401881. [DOI] [PubMed] [Google Scholar]
- 15.Sakaki S, Matsuo Y, Kuwabara H, Matsuoka K. Rupture of an aneurysm into parasellar epidermoid cyst. J Neurosurg. 1981;55:629–632. doi: 10.3171/jns.1981.55.4.0629. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki T, Wakai S, Asano T, Watanabe T, Kirino T, Sano K. The effect of a lipid hydroperoxide of arachidonic acid on the canine basilar artery. An experimental study on cerebral vasospasm. J Neurosurg. 1981;54:357–365. doi: 10.3171/jns.1981.54.3.0357. [DOI] [PubMed] [Google Scholar]
- 17.Schenk VW, Solleveld H. Multiple aneurysms in a case of acromegaly. Psychiatr Neurol Neurochir. 1968;71:311–317. [PubMed] [Google Scholar]
- 18.Stephenson TF, Spitzer RM. MR and CT appearance of ruptured intracranial dermoid tumors. Comput Radiol. 1987;11:249–251. doi: 10.1016/0730-4862(87)90007-2. [DOI] [PubMed] [Google Scholar]
- 19.Wakai S, Fukushima T, Furihata T, Sano K. Association of cerebral aneurysm with pituitary adenoma. Surg Neurol. 1979;12:503–507. [PubMed] [Google Scholar]
- 20.Stendel R, Pietilä TA, Lehmann K, Kurth R, Suess O, Brock M. Ruptured intracranial dermoid cysts. Surg Neurol. 2002;57:391–398. doi: 10.1016/s0090-3019(02)00723-1. discussion 398. [DOI] [PubMed] [Google Scholar]