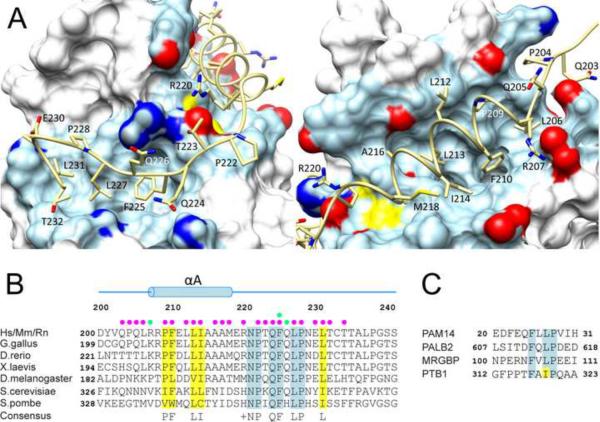
Figure 3.
The MRG15-Pf1 intermolecular interface and conservation of a sequence motif in MRG binders. (A) Non-covalent interactions at the MRG15 MRG-Pf1 MBD interface. The MRG domain is rendered as a molecular surface with residues making contacts with Pf1 shown in light blue with the side chain oxygen, nitrogen and sulfur atoms colored in red, blue and yellow, respectively. (B) A MEME-guided multiple sequence alignment of the MRG-binding domain of Pf1 orthologs. Species abbreviations: Hs: Homo sapiens; Mm: Mus musculus; Rn: Rattus norvegicus. Conserved and invariant residues are highlighted in yellow and blue, respectively. Filled circles denote intermolecular hydrophobic (magenta) and hydrogen bonding (green) interactions in the MRG15-Pf1 complex. (C) A multiple sequence alignment of various MRG interactors that harbor FxLP motifs in their MRG-binding domains. See also Figure S5.