FIGURE 4.
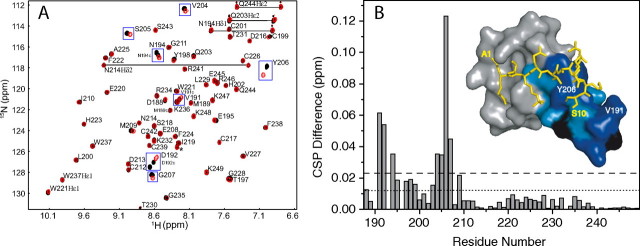
Binding of ING4-PHD to H3K4me3 peptides of different lengths. A, overlay of HSQC spectra of ING4(188–249) bound to H315K4me3 (black) or H310K4me3 (red). The cross-peaks are labeled with their corresponding residue number and single-letter code. Peaks from amide side chains are connected by straight lines. Residues with CSP differences larger than twice the experimental error are boxed. Four signals from a minor conformer caused by the cis-trans isomerization of the peptidyl-prolyl bonds in the flexible N-terminal region of ING4 are labeled (M189c, V191c, D192c, and N194c). A signal from an unknown molecule is marked with an asterisk. B, binding histogram of the differences in the CSP for each ING4 PHD residue; the experimental error (±0.012 ppm) is indicated by the dotted line, while twice this error is indicated by the dashed line. The inset is a surface representation of ING4 PHD where residues with 0 > ΔCSP > error or ΔCSP > 2 × error are colored in light or dark blue, respectively. Proline residues for which no signal can be observed are colored in black.