FIGURE 1.
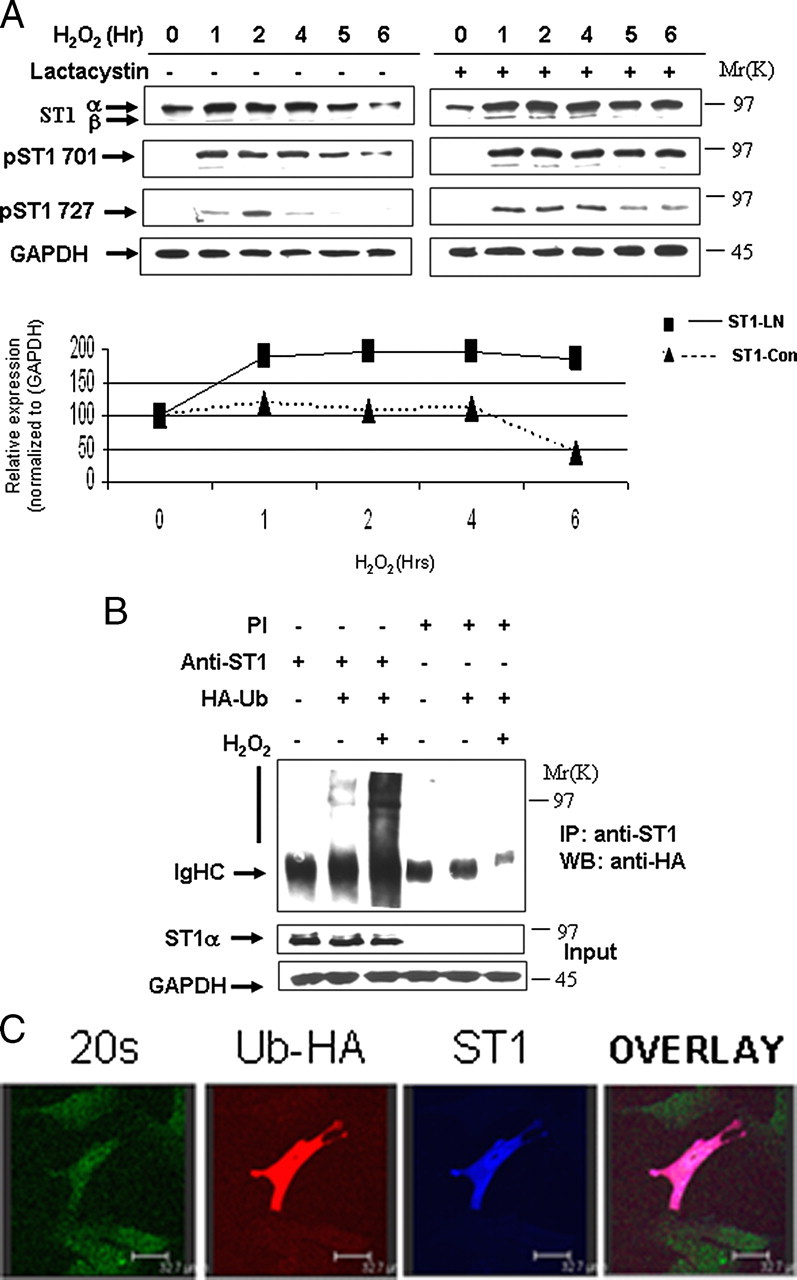
STAT1 is ubiquitylated and degraded in a proteasome-dependent manner. A, H2O2-mediated oxidative stress leads to STAT1 degradation (left panel). MEF cells were exposed to H2O2 (200 μm) at the indicated times and cell lysates analyzed by Western blotting with the indicated antibodies, anti-STAT1α or STAT1β (E-23) (ST1α,-β), anti-STAT1 phosphotyrosine (pST1 701), or phosphoserine (ST1 727). Similar experiments were performed as in A in the presence of the proteasomal inhibitor lactacystin (10 μm) (ST1-LN) or without (ST-con) (bottom panel). The results shown in the right panel were subjected to densitometric analysis, and results were normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression levels and shown in graph form. Similar results were observed in three independent experiments. B, STAT1 ubiquitylation is enhanced in MEF cells exposed to oxidative stress. MEF cells were transfected with control vector or an HA-tagged ubiquitin (HA-Ub) expression construct and exposed to H2O2 (200 μm) for 4 h. Cell lysates were immunoprecipitated (IP) either with anti-STAT1α (E-23) (anti-ST1) or preimmune (PI) control antibodies, and Western blots (WB) were performed as indicated. STAT1α (E-23) (ST1α). C, ubiquitylated STAT1 co-localizes with the 20 S proteasomal following H2O2. MEF cells were plated on coverslips and transfected with Myc-tagged STAT1 and HA-tagged ubiquitin and exposed to H2O2 (200 μm) for 4 h in the presence of the proteasome inhibitor MG132 (20 μm). Cells were stained with anti-Myc (ST1) blue, anti-HA (ubiquitin) red, and anti-20 S proteasome (20 S) green.
