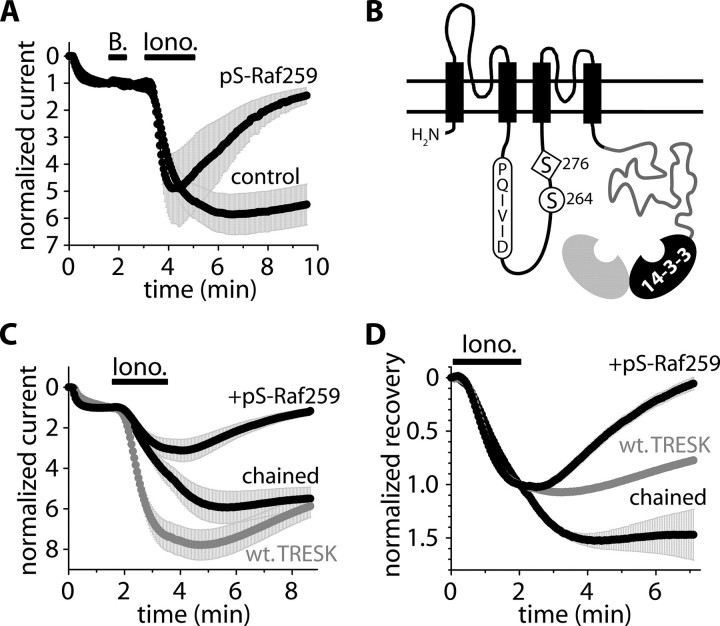
FIGURE 3.
The 14-3-3 inhibitor Ser(P)-Raf259 accelerates the recovery of TRESK and the chained construct. A, normalized currents of oocytes microinjected with the phosphopeptide (pS-Raf259, 50 nl, 10 mm, 37–112 min before the application of ionomycin) or with water (control). The cells were coexpressing mouse TRESK with 14-3-3η (experimental protocol and current normalization were described in the legend to Fig. 1). B, schematic topology of the chained construct. TRESK was connected to 14-3-3η via the custom-designed, flexible polypeptide chain (gray). The NFAT-like calcineurin binding motif (PQIVID), serine 264, and 276 were also depicted. The chained 14-3-3η (black) is shown to be dimerized with another 14-3-3 subunit (gray) deriving from the other chained construct (of the functional TRESK dimer; not shown) or from the endogenous pool of the oocyte. C and D, normalized currents and recoveries of the oocytes expressing wild type TRESK (wt. TRESK, gray) or the chained construct. The cells expressing the chained construct were microinjected with the phosphopeptide (+pS-Raf259, 50 nl, 10 mm, 37–156 min before the application of ionomycin) or with water (chained).