FIGURE 5.
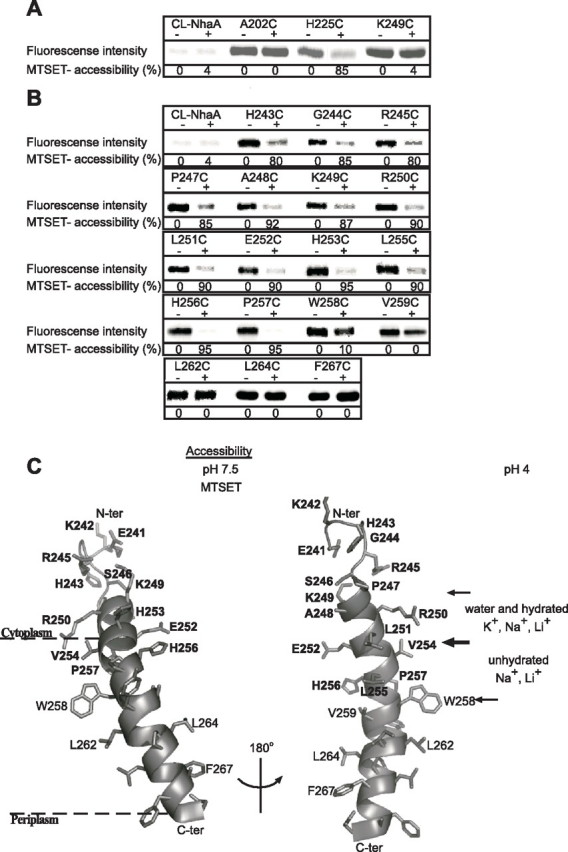
Accessibility of Cys replacements in loop VIII–IX and TMS IX to MTSET. Intact EP432 cells (A) expressing the various NhaA mutants from pCL-HAH4 and everted membrane vesicles isolated from them (B) were incubated with MTSET (+) or were untreated (-) as described under “Experimental Procedures.” Proteins were purified by Ni2+-NTA affinity chromatography and labeled on the beads with the fluorescent reagent fluorescein-5-maleimide to estimate the percentage of free cysteines left. Then the eluted proteins were resolved by SDS-PAGE, and the fluorescence intensity on the gels was determined by a phosphorimaging system (Fuji Bas 1000). The same gels were also analyzed for the protein amounts using Coomassie Blue staining (not shown) and measuring the densities of the bands. The fluorescence intensities were normalized according to the amount of the proteins and expressed as a percentage of the untreated control (100%). Accessibility to MTSET = 100% - the percentage of fluorescein-5-maleimide labeling. CL-NhaA served as a negative control indicating the specificity of the reagents to SH groups. Several concentrations of the reagent were tested with similar results. The standard deviation was between 5 and 10%. C, a ribbon representation of TMS IX and the C-terminal part of loop VIII–IX. The single letter amino acid codes are used, and the location in the amino acid sequence is indicated by a number. Amino acid residues of which Cys replacements are accessible to MTSET at pH 7.5 are shown in white on black rectangles. Based on the crystal structure at pH 4 (11), the borders of the cytoplasmic funnel are depicted by thin arrows. The sections of the funnel above and below Glu252 (thick arrow) mark the orifice of the cytoplasmic funnel and the narrow passage into the middle of the membrane with their potential accessibility to ions at pH 4, respectively. The representation was generated using PyMOL. ter, terminus.
