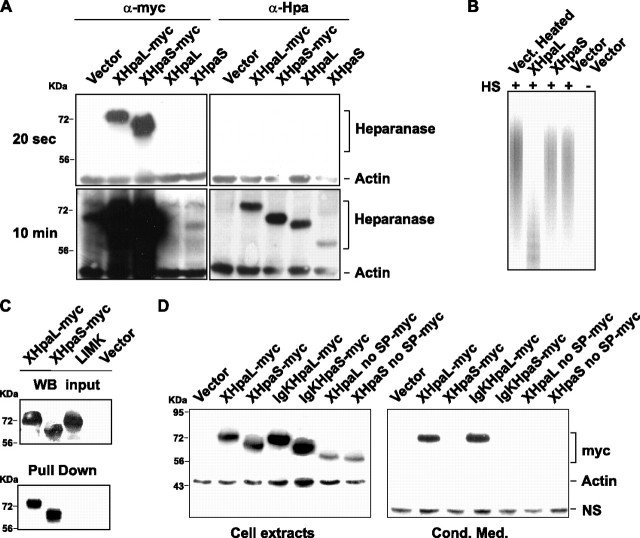
FIGURE 2.
XHpaL and XHpaS differ in their secretion and endoglucuronidase properties. A, expression of XHpaL and XHpaS. COS-7 cells were transfected with heparanase XHpaL and XHpaS constructs with or without the myc tag. After 48 h, 40 μg of cell extract were analyzed by Western blot using anti-myc or anti-human heparanase (Clone HP3/17) antibodies.β-Actin detection was used as equal loading control. The same Western blot was exposed 20 s (top) to detect the myc tags and 10 min (bottom) for detection with the anti-heparanase antibody. B, heparanase activity of XHpaL. COS-7 cells were transfected with XHpaL, XHpaS, or mock control (vector). One day after transfection, cell extracts were incubated with FITC-HS for an additional 24 h and degradation products of FITC-HS were detected on a native, 15% polyacrylamide gel. C, XHpaL and XHpaS interact with heparin. Cellular extracts from COS-7 cells transiently transfected with myc-tagged XHpaL, XHpaS, and LIMK1 were incubated with heparin-acrylic beads. Bound proteins were evaluated by Western blotting with anti-myc antibodies. A Western analysis of myc-tagged proteins from input is also shown. D, secretion of XHpaL but not XHpaS. COS-7 cells were transfected with the empty vector, constructs expressing myc-tagged XHpaL and XHpaS, or constructs expressing Xenopus heparanases with a deleted signal peptide (XHpaL and XHpaS no SP) or with the Xenopus signal peptide replaced with the human immunoglobulin κ leader sequence (IgKHpaL and IgKHpaS). After 24 h, cell extracts and conditioned medium were analyzed by Western blotting utilizing anti-myc antibodies. β-Actin in the cell extract and the presence of a nonspecific band (NS) in conditioned medium were used as equal loading controls.