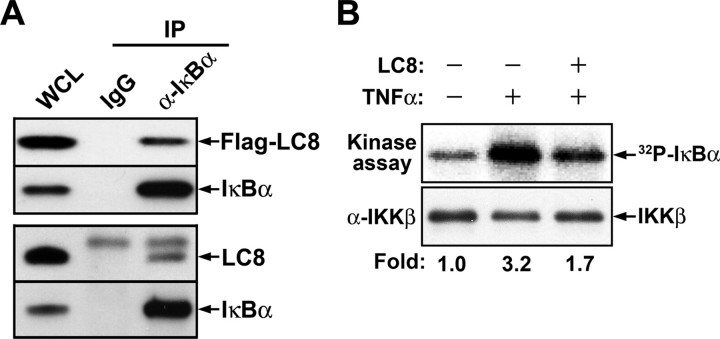
FIGURE 1.
Interaction of LC8 with IκBα and its inhibition of IκBα phosphorylation by IKK. A, association of LC8 with IκBα. HeLa cells transfected for 24 h with pFLAG-LC8 were lysed in phosphate-buffered saline containing 1 mm EDTA, 0.5% Nonidet P-40, 1 mm AEBSF, aprotinin (10 μg/ml), and leupeptin (10 μg/ml). The cell lysates were incubated with normal rabbit IgG or antibody to IκBα (α-IκBα) for 2 h at 4 °C, and the immune complexes were then precipitated with protein A-Sepharose. Whole cell lysate (WCL) and immunoprecipitates (IP) were subjected to immunoblot analysis with antibodies to FLAG or to IκBα (upper panel). Alternatively, lysates of nontransfected HeLa cells were similarly subjected to immunoprecipitation, and endogenous LC8 was detected with antibody to LC8 (lower panel). B, inhibition by LC8 of IκBα phosphorylation mediated by IKK in vitro. An in vitro kinase assay was performed with recombinant IκBα, with IKK immunoprecipitated from cells treated (or not) with TNFα, and in the absence or presence of LC8, as described under “Experimental Procedures”. The relative activities of IKK were determined from the radioactivity (32P) associated with IκBα bands. The reaction mixtures were also subjected to immunoblot analysis with antibody to IKKβ.