FIGURE 1.
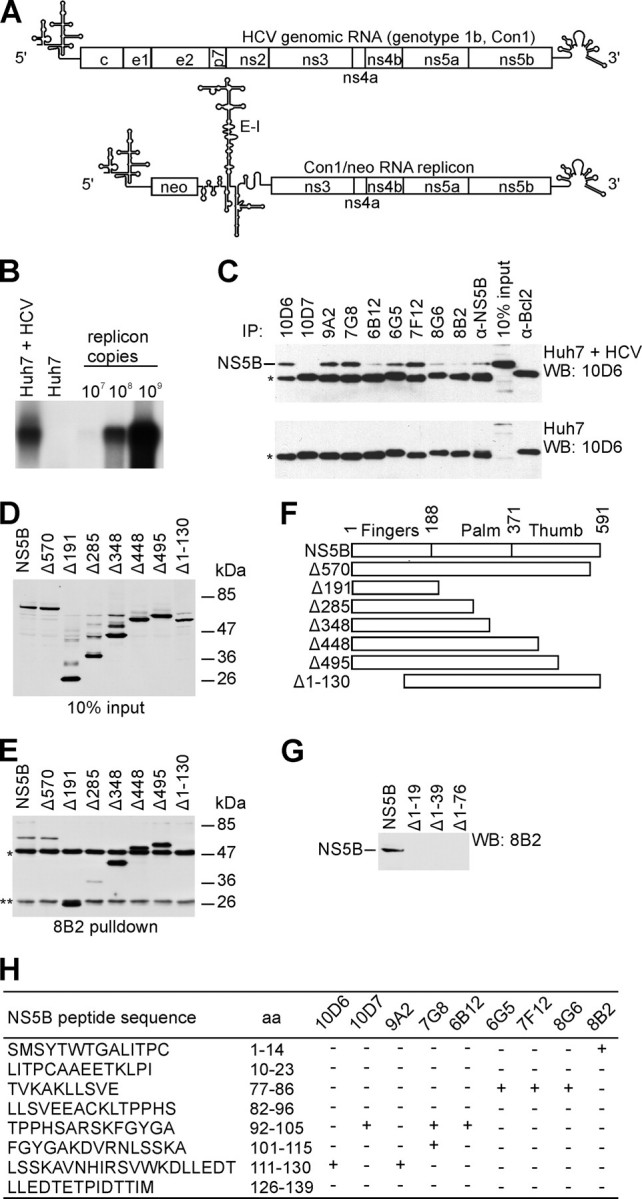
Features of mAbs directed against HCV NS5B RdRp. A, schematic representation of HCV genomic RNA and subgenomic replicon Con1/neo construct (25, 29) used for stable cell line selection (5′, HCV 5′-UTR; neo, neomycin phosphotransferase gene; E-I, encephalomyocarditis virus IRES; 3′, HCV 3′-UTR). Reported secondary structures for 5′-UTR (60), 3′-UTR (61), and encephalomyocarditis virus IRES (62) were used in the illustration. B, detection of Con1/neo subgenomic replicon in G418 selected Huh7 cells. Northern blot analysis was performed using total cellular RNA and antisense ns5b-specific probe. 32P-Labeled Con1/neo RNA was used as a marker. C, immunoprecipitation (IP)/Western blot (WB) analysis. Various mAbs were used to immunoprecipitate the NS5B either from the lysate of the Huh7 cell line containing selectable subgenomic HCV RNA replicon depicted in A (Huh7 + HCV) or from the Huh7 cell line lysate (Huh7). NS5B polyclonal antibodies (α-NS5B) and α-Bcl2 mAb, directed against cellular protein, were used as positive and negative controls, respectively. *, immunoglobulin heavy chain; protein blots were probed with NS5B-specific mAb 10D6, and reactivity was detected with secondary horseradish peroxidase-conjugated antibody of a murine origin. D, Western blot analysis of bovine papilloma virus E2 protein 3F12 epitope-tagged (63) NS5B truncated construct expression in COS7 cells. Molecular mass standards are indicated on the right. E, immunoprecipitation of the proteins shown in D using mAb 8B2. **, immunoglobulin light chain; protein blots were probed with bovine papilloma virus E2-specific mAb 3F12, and reactivity was detected as in C. F, schematic diagram of wild-type HCV NS5B (shown on top) and deletion constructs used for immunoprecipitation. Polymerase subdomains are indicated. Numbers refer to the first residue in a given subdomain. G, Western blot analysis of mAb 8B2 binding to N-terminal truncations of the NS5B protein. H, results of the epitope mapping for NS5B mAbs. Wells of MaxiSorp™ plate (Nunc) were coated with 5 μg of peptide and blocked with bovine serum albumin. HCV NS5B-specific mAbs were incubated with the peptides, and ELISA reactivity was detected by horseradish peroxidase-conjugated antibody; aa, amino acid residues.
