FIGURE 2.
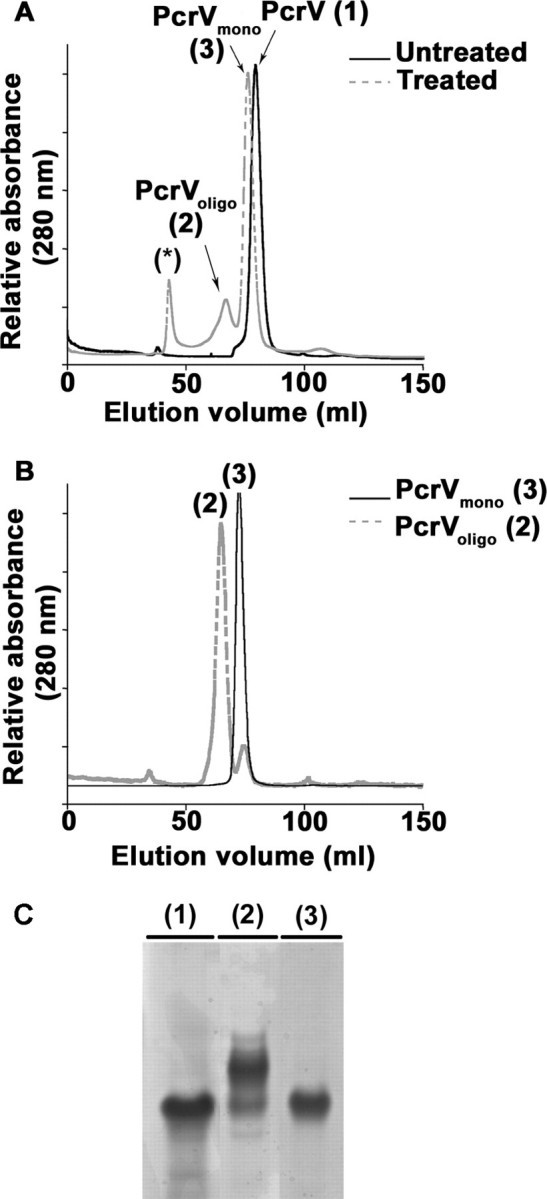
Formation of novel, oligomeric species of PcrV. A, SEC analysis of pH-treated and untreated PcrV protein shows the formation of two novel protein peaks after refolding. Untreated PcrV was recovered as a single peak corresponding to a monomer (PcrVuntreated (1)). After pH treatment, two novel peaks arise in addition to the one corresponding to the monomer (PcrVmono (3)). One peak corresponds to multimeric species (PcrVoligo (2)) and the other probably to aggregated species eluting in void volume (asterisk). B, proteins present either in peak 2 (PcrVoligo) or peak 3 (PcrVmono) were analyzed again by SEC showing that the oligomers and monomers are stable species. C, the different protein peaks were submitted to native gel electrophoresis followed by Coomassie Blue staining. Lanes 1-3 correspond to 10 μg of protein contained in the peaks eluted from SEC: PcrVuntreated, PcrVoligo, and PcrVmono, respectively. PcrVuntreated (1) runs as a unique band as does the PcrVmono (3), whereas PcrVoligo (2) separates into additional bands with lower electrophoretic mobilities.
