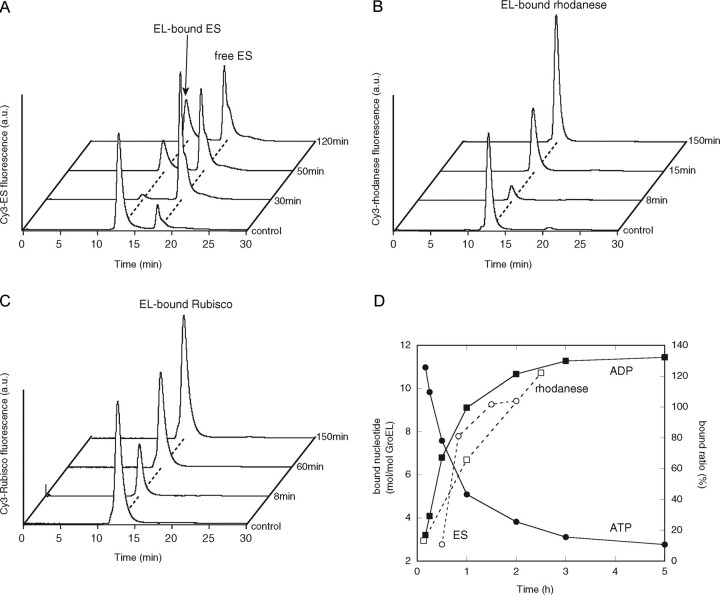
FIGURE 4.
Binding of GroES and the denatured substrate to the symmetric GroES-EL398-GroES ATP football complex. A, binding of Cy3-GroES to the EL398 ATP football complex formed using unlabeled GroES. The stable EL398 ATP football complex was generated by mixing EL398 (EL) with GroES (ES) at a 1:2 molar ratio in the presence of ATP (t = 0 min) and incubating the mixture for 5 min. The mixture was rapidly applied to a gel filtration column to isolate the ATP football complex. The isolated ATP football complex (0.5 μm) was mixed with Cy3-GroES (1 μm) and ATP (1 mm) at the indicated times and then separated by gel filtration HPLC with fluorescence detection. B and C, binding of denatured Cy3-labeled substrates to the EL398 ATP football complex. The ATP football complexes (0.5 μm) were mixed with either Cy3-rhodanese (0.25 μm)(B) or Cy3-Rubisco (0.25 μm)(C) at the indicated times and then separated by gel filtration with fluorescence detection. The trace labeled control represents the direct mixing of the empty EL398 (0.5 μm) with 1 μm Cy3-GroES and 1 mm ATP (A), 0.25 μm Cy3-rhodanese (B), or 0.25 μm Cy3-Rubisco (C). a.u., arbitrary units. D, time course of ATP hydrolysis by the isolated EL398 ATP football complex and quantification of the GroES and rhodanese bound to the EL398 ATP football complexes. The hydrolysis of ATP in the isolated ATP football complexes was determined as described under “Experimental Procedures.” The determination of the amounts of bound GroES and rhodanese was performed as follows. The integrated peak areas in the traces obtained by the binding assay of Cy3-GroES (A) and Cy3-rhodanese (B) were calculated. The amounts of GroES and rhodanese bound to unliganded EL398 (control in A and B) were taken as 200 and 100%, respectively.